Atorvastatin calcium is a synthetic lipid-lowering agent. Atorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, an early and rate-limiting step in cholesterol biosynthesis.
Atorvastatin, as well as some of its metabolites, are pharmacologically active in humans. The liver is the primary site of action and the principal site of cholesterol synthesis and LDL clearance. Drug dosage, rather than systemic drug concentration, correlates better with LDL-C reduction. Individualization of drug dosage should be based on therapeutic response.
Absorption: Atorvastatin is rapidly absorbed after oral administration, with maximum plasma concentrations occurring within 1 to 2 hours. The extent of absorption increases in proportion to the Atorvastatin dose. The absolute bioavailability of Atorvastatin (parent drug) is approximately 14%, and the systemic availability of HMG-CoA reductase inhibitory activity is approximately 30%.
Distribution: The mean volume of distribution of Atorvastatin is approximately 381 liters. Atorvastatin is 98% bound to plasma proteins. A blood/plasma ratio of approximately 0.25 indicates poor drug penetration into red blood cells. Based on observations in rats, Atorvastatin is likely to be secreted in human milk.
Metabolism: Atorvastatin is extensively metabolized to ortho- and parahydroxylated derivatives and various beta-oxidation products. In vitro inhibition of HMG-CoA reductase by ortho- and parahydroxylated metabolites is equivalent to that of Atorvastatin. Approximately 70% of circulating inhibitory activity for HMG-CoA reductase is attributed to active metabolites. In vitro studies suggest the importance of Atorvastatin metabolism by cytochrome P450 3A4, which is consistent with increased plasma concentrations of Atorvastatin in humans following co-administration with erythromycin, a known inhibitor of this isozyme. In animals, the ortho-hydroxy metabolite undergoes further glucuronidation.
Excretion: Atorvastatin and its metabolites are eliminated primarily in bile following hepatic and/or extra-hepatic metabolism. However, the drug does not appear to undergo enterohepatic recirculation. The mean plasma elimination half-life of Atorvastatin in humans is approximately 14 hours, but the half-life of inhibitory activity for HMG-CoA reductase is 20 to 30 hours due to the contribution of active metabolites. Less than 2% of a dose of Atorvastatin is recovered in urine following oral administration.
Geriatric: Plasma concentrations of Atorvastatin are higher (approximately 40% for Cmax and 30% for AUC) in healthy elderly subjects (aged 65 years) than in young adults. Clinical data suggest a greater degree of LDL-lowering at any dose of the drug in the elderly patient population compared to younger adults.
Pediatric: Pharmacokinetic data in the pediatric population are not available.
Gender: There is no clinically significant difference in LDL-C reduction with Atorvastatin between men and women.
Renal Insufficiency: Renal disease has no influence on the plasma concentrations or LDL-C reduction of Atorvastatin. Thus, dose adjustment in patients with renal dysfunction is not necessary.
Hemodialysis: Hemodialysis is not expected to significantly enhance the clearance of Atorvastatin since the drug is extensively bound to plasma proteins.
Hepatic Insufficiency: In patients with chronic alcoholic liver disease, plasma concentrations of Atorvastatin are markedly increased.

Atorvastatin is indicated:
As an adjunct to diet to reduce elevated total cholesterol, LDL-C, apolipoprotein B, and triglyceride (TG) levels, and to increase HDL-C in patients with primary hypercholesterolemia and mixed dyslipidemia.
As an adjunct to diet for the treatment of patients with elevated serum TG levels.
For the treatment of patients with primary dysbetalipoproteinemia who do not respond adequately to diet.
To reduce total cholesterol and LDL-C in patients with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering treatments.
As an adjunct to diet to reduce total cholesterol, LDL-C, and apolipoprotein B levels in boys and postmenarchal girls, 10 to 17 years of age, with heterozygous familial hypercholesterolemia.
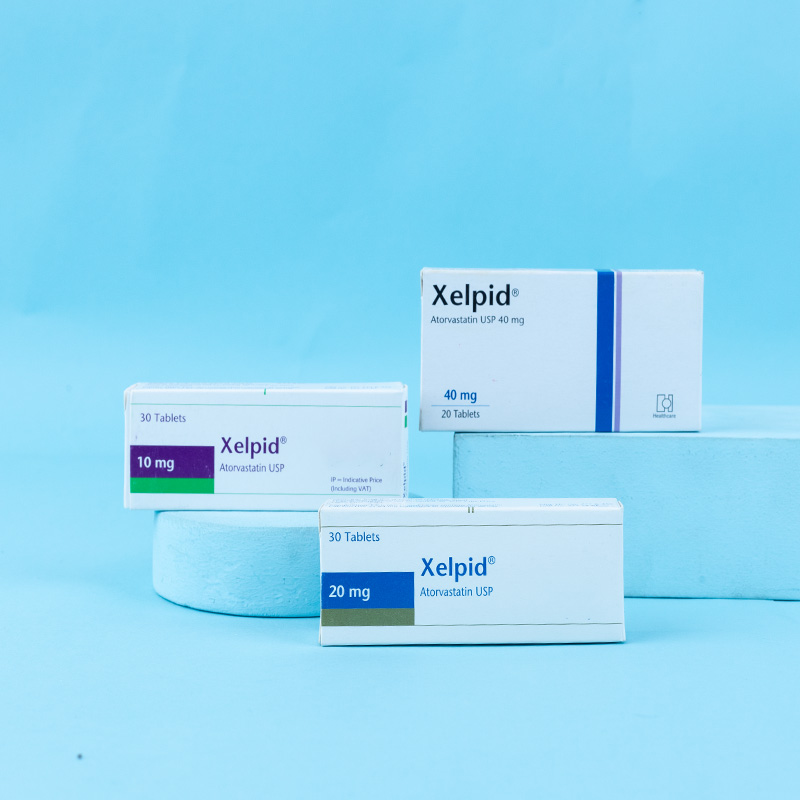
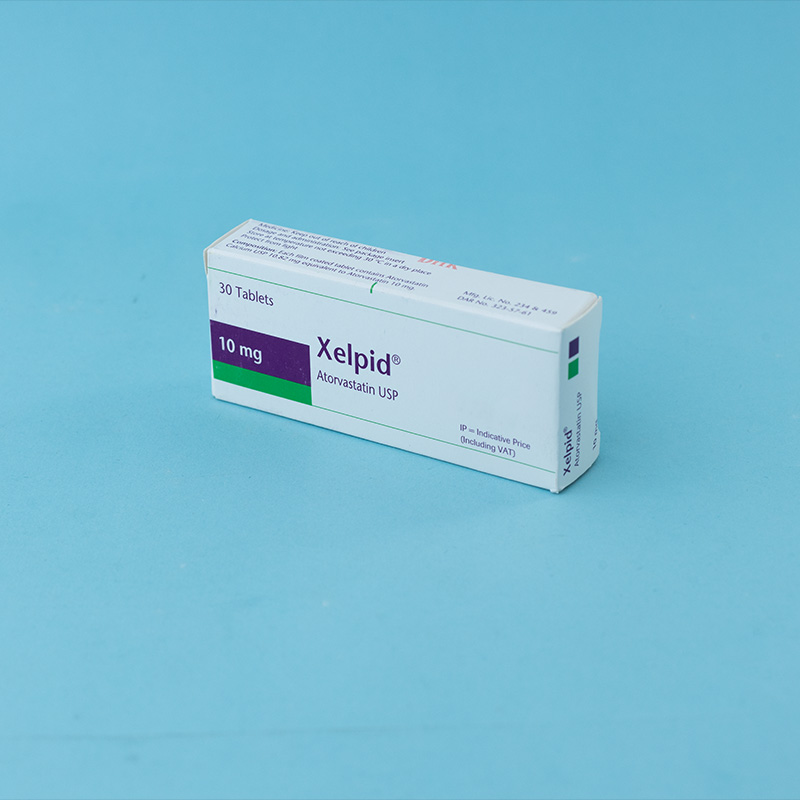
The patient should be placed on a standard cholesterol-lowering diet before receiving Atorvastatin and should continue on this diet during treatment. The recommended starting dose of Atorvastatin is 10 or 20 mg once daily. Patients who require a large reduction in LDL-C (more than 45%) may be started at 40 mg once daily. The dosage range of Atorvastatin is 10 to 80 mg once daily. Atorvastatin can be administered as a single dose at any time of the day, with or without food. After initiation of Atorvastatin, lipid levels should be analyzed within 2 to 4 weeks, and the dosage adjusted accordingly.
In pediatric patients (10-17 years of age) with hypercholesterolemia, the recommended starting dose of Atorvastatin is 10 mg/day, with a maximum recommended dose of 20 mg/day (doses greater than 20 mg have not been studied in this patient population). Doses should be individualized according to the recommended goal of therapy. Adjustments should be made at intervals of 4 weeks or more.
Active liver disease or unexplained persistent elevations of serum transaminases. Hypersensitivity to any component of this medication.
Before initiating therapy with Atorvastatin, an attempt should be made to control hypercholesterolemia with appropriate diet, exercise, and weight reduction in obese patients, as well as to treat other underlying medical problems.
HMG-CoA reductase inhibitors are contraindicated during pregnancy and in nursing mothers. ATORVASTATIN SHOULD BE ADMINISTERED TO WOMEN OF CHILDBEARING AGE ONLY WHEN SUCH PATIENTS ARE HIGHLY UNLIKELY TO CONCEIVE AND HAVE BEEN INFORMED OF THE POTENTIAL HAZARDS. If the patient becomes pregnant while taking this drug, therapy should be discontinued, and the patient apprised of the potential hazard to the fetus.
Atorvastatin is generally well tolerated. The most frequent adverse events thought to be related to Atorvastatin include constipation, flatulence, dyspepsia, and abdominal pain.
Cyclosporine, fibric acid derivatives, niacin, erythromycin, azole antifungals: Increased risk of myopathy.
Antacid: Plasma concentrations of Atorvastatin decreased by approximately 35%, but LDL-C reduction was not altered.
Colestipol: Plasma concentrations of Atorvastatin decreased by approximately 25%, but LDL-C reduction was greater when both drugs were coadministered.
Digoxin: Steady-state plasma digoxin concentrations increased by approximately 20%. Monitoring is recommended.
Oral contraceptives: Increased AUC values for norethindrone and ethinyl estradiol by approximately 30% and 20%, respectively.
Warfarin: No clinically significant effect on prothrombin time.
There is no specific treatment for Atorvastatin overdosage. Supportive measures should be instituted as required. Due to extensive drug binding to plasma proteins, hemodialysis is not expected to significantly enhance clearance.
Store at a temperature not exceeding 30°C in a dry place. Protect from light.
Medicine: Keep out of reach of children.