Prasugrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.

Prasugrel is a P2Y12 platelet inhibitor indicated for the reduction of thrombotic cardiovascular events (including stent thrombosis) in patients with acute coronary syndrome who are to be managed with PCI, as follows:
● Patients with unstable angina or non-ST-elevation myocardial infarction (NSTEMI)
● Patients with ST-elevation myocardial infarction (STEMI)

Initiate treatment with a single 60 mg oral loading dose. Continue with 10 mg once daily, with or without food. Consider 5 mg once daily for patients weighing less than 60 kg. Patients should also take aspirin (75 mg to 325 mg) daily.
Bleeding, including life-threatening and fatal bleeding, is the most commonly reported adverse reaction. Thrombotic thrombocytopenic purpura (TTP) is also a known adverse reaction.
Co-administration with warfarin or NSAIDs (when used chronically) may increase the risk of bleeding.
● CABG-related bleeding: The risk increases in patients receiving prasugrel who undergo CABG.
● Discontinuation of prasugrel: Premature discontinuation increases the risk of stent thrombosis, myocardial infarction, and death.
● Thrombotic thrombocytopenic purpura (TTP): TTP has been reported with prasugrel use.
Pregnancy: Pregnancy Category B. There are no adequate and well-controlled studies of prasugrel use in pregnant women. Prasugrel should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
Nursing Mothers: It is not known whether prasugrel is excreted in human milk. Because many drugs are excreted in human milk, prasugrel should be used during nursing only if the potential benefit to the mother justifies the potential risk to the nursing infant.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Geriatric Use: Due to the risk of bleeding and uncertain effectiveness in patients 75 years of age or older, prasugrel use is generally not recommended in this population, except in high-risk situations (such as diabetes and a history of myocardial infarction), where its benefits appear to be greater and its use may be considered.
Low Body Weight: Individuals with a body weight of less than 60 kg have an increased risk of bleeding. Consider lowering the maintenance dose to 5 mg in such patients.
Store in a cool and dry place below 30°C. Medicine: Keep out of reach of children

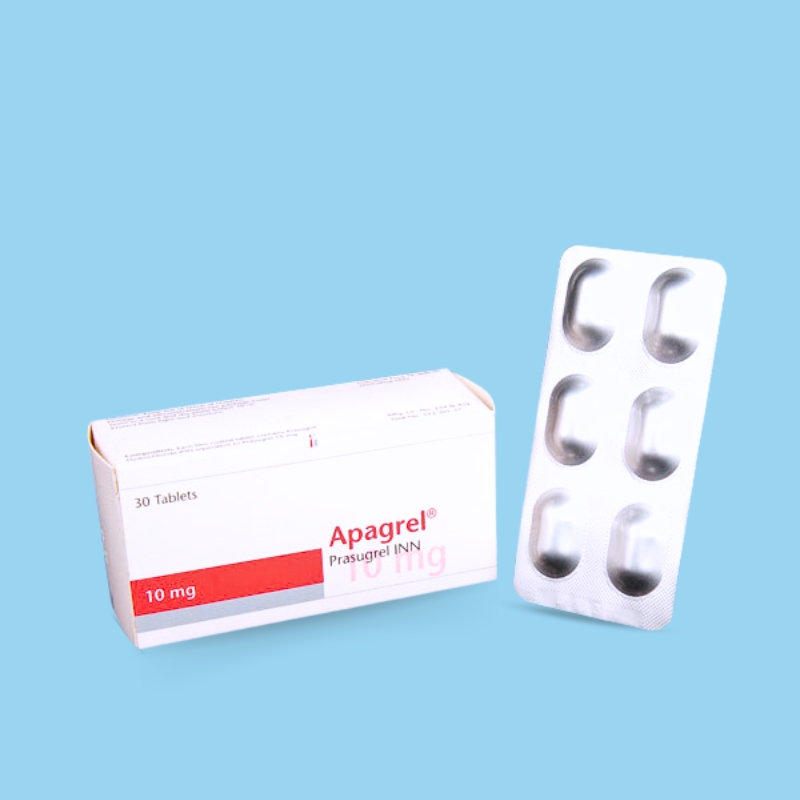
.jpg)