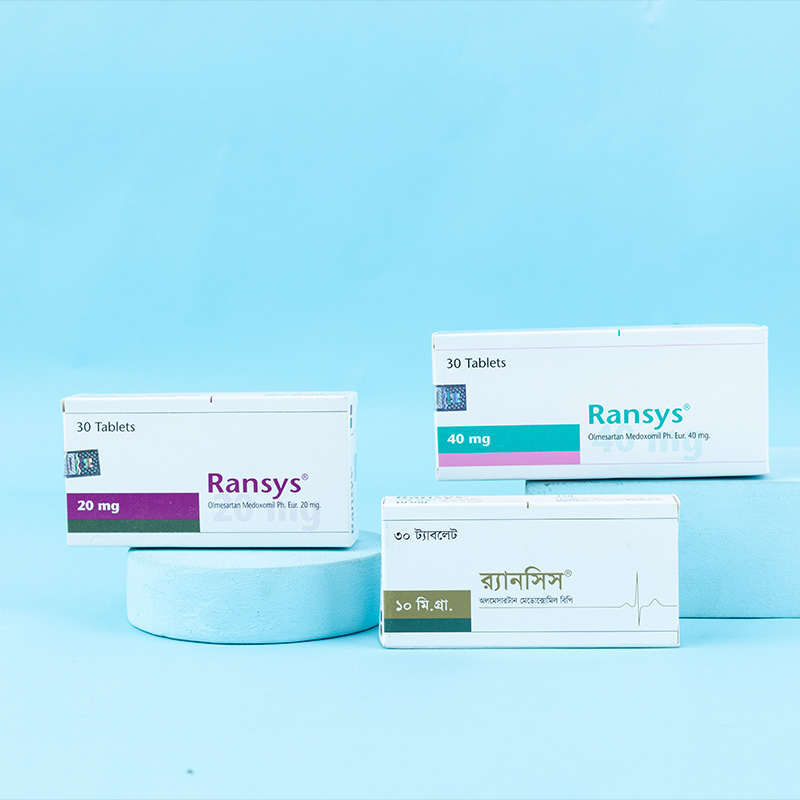
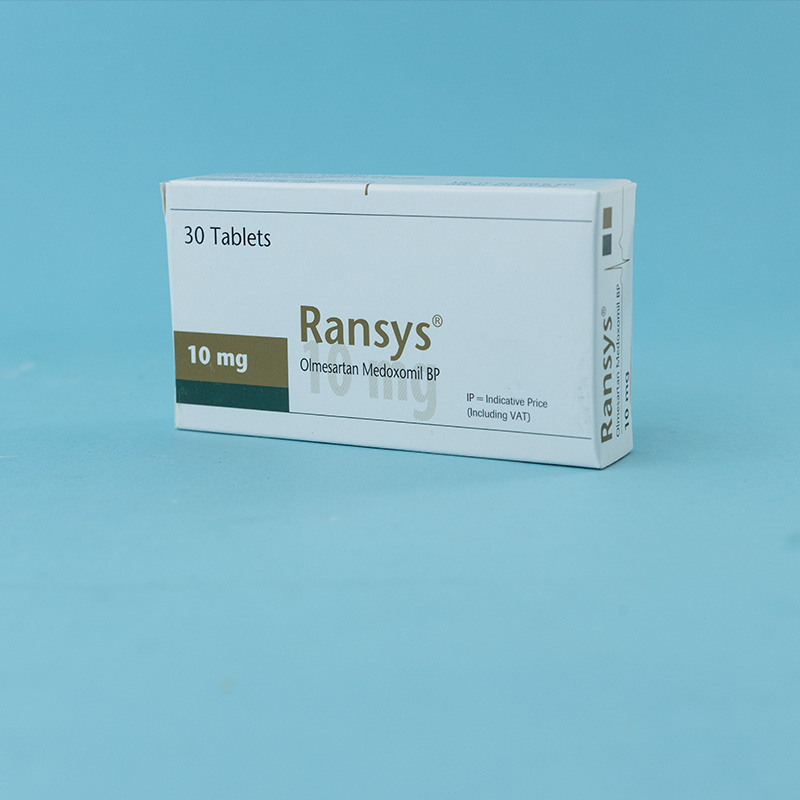
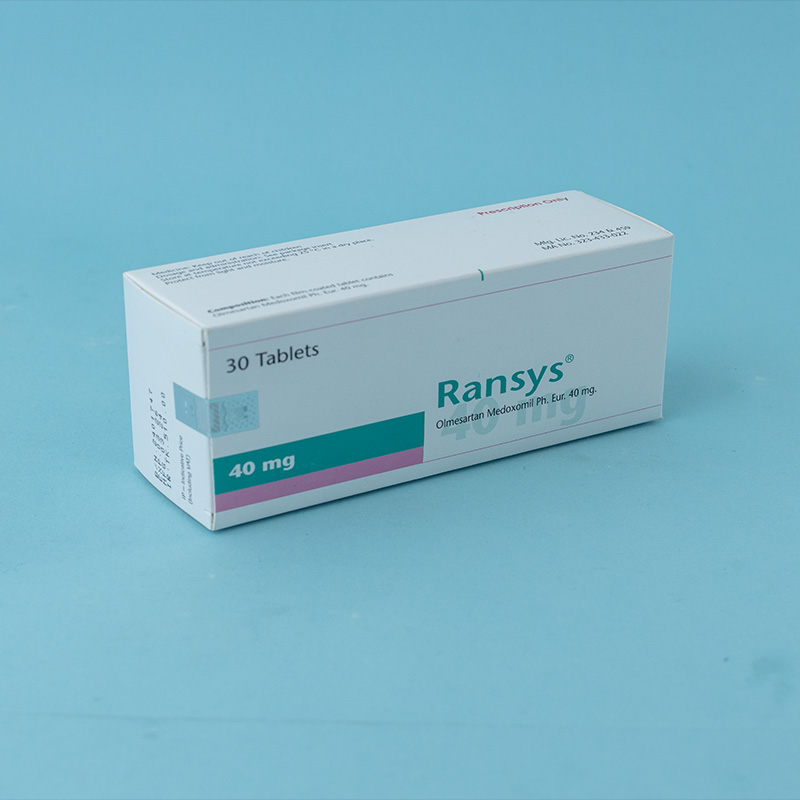
Olmesartan medoxomil is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
Dosage must be individualized. The usual recommended starting dose of Olmesartan is 20 mg once daily when used as monotherapy in patients who are not volume-contracted. For patients requiring further blood pressure reduction after two weeks of therapy, the dose may be increased to 40 mg once daily. Doses above 40 mg do not appear to provide additional benefits. Twice-daily dosing offers no advantage over a once-daily total dose.
No initial dosage adjustment is required for elderly patients, those with moderate to marked renal impairment (creatinine clearance < 40 ml/min), or those with moderate to marked hepatic dysfunction. However, for patients at risk of intravascular volume depletion (e.g., those on diuretics, particularly those with impaired renal function), Olmesartan should be initiated under close medical supervision, and a lower starting dose should be considered. Olmesartan may be taken with or without food.
Treatment with Olmesartan is generally well tolerated, with an incidence of adverse events similar to placebo. In placebo-controlled clinical trials, the following adverse events occurred in more than 1% of patients treated with Olmesartan but also occurred at a similar or greater incidence in placebo-treated patients: back pain, bronchitis, increased creatine phosphokinase, diarrhea, headache, hematuria, hyperglycemia, hypertriglyceridemia, influenza-like symptoms, pharyngitis, rhinitis, and sinusitis.
Olmesartan is contraindicated in patients who are hypersensitive to any component of this product.
Pregnancy Categories: C (first trimester) and D (second and third trimesters).
Nursing Mothers: It is unknown whether Olmesartan is excreted in human milk, but it has been detected in low concentrations in the milk of lactating rats. Due to the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, considering the drug’s importance to the mother.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Serum electrolytes should be periodically monitored to detect possible imbalances.
Olmesartan must be discontinued as soon as pregnancy is detected.
Hypotension may occur in volume- or salt-depleted patients.
Caution is advised in patients with impaired renal function.
Store at a temperature not exceeding 25ºC in a dry place, protected from light.
Medicine: Keep out of reach of children.