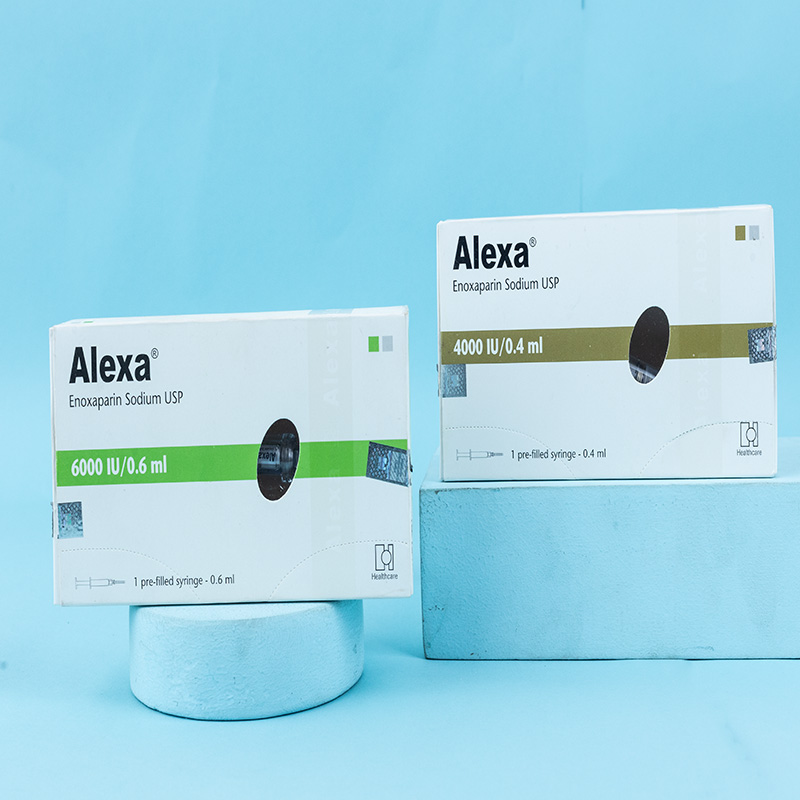
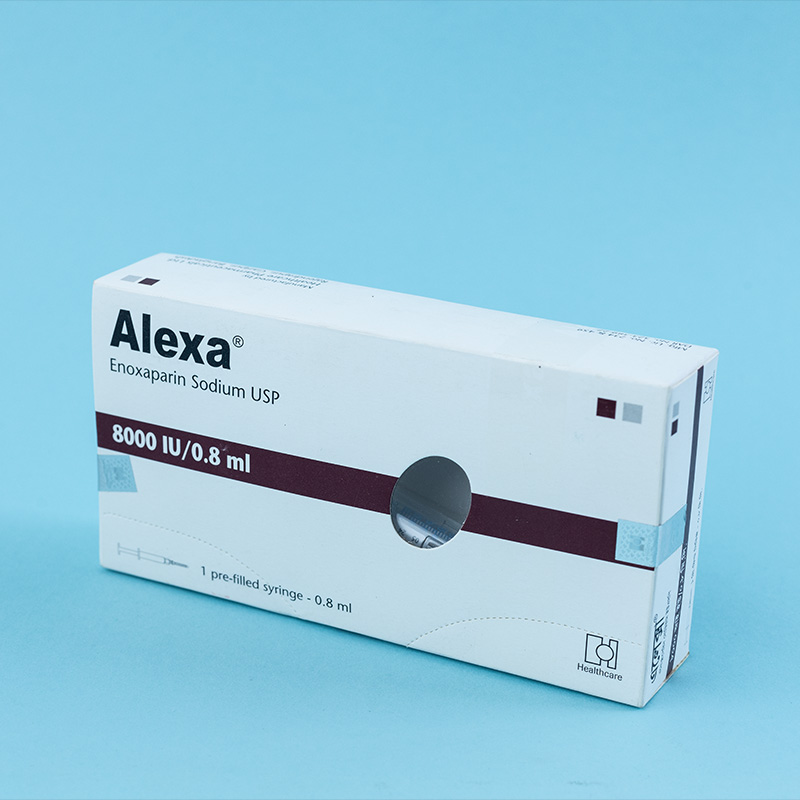
Enoxaparin is indicated for:
Treatment of deep vein thrombosis, with or without pulmonary embolism.
Treatment of unstable angina and non-Q-wave myocardial infarction, administered concurrently with aspirin.
Prevention of thrombus formation in the extracorporeal circulation during haemodialysis.
Prophylaxis of venous thromboembolic disease (prevention of blood clot formation in the veins), particularly those associated with orthopedic or general surgery.
Prophylaxis of venous thromboembolic disease in medical patients bedridden due to acute illness, including cardiac insufficiency, respiratory failure, severe infections, and rheumatic diseases.
Indication and Recommended Dosage Schedule
Treatment of deep vein thrombosis, with or without pulmonary embolism:
Subcutaneous injection of 100 IU/kg twice daily for 10 days, or
Subcutaneous injection of 150 IU/kg once daily for 10 days.
Note: Oral anticoagulant therapy should be initiated when appropriate, and Enoxaparin treatment should be continued until a therapeutic anticoagulant effect has been achieved.
Treatment of unstable angina and non-Q-wave myocardial infarction, administered concurrently with aspirin: Subcutaneous injection of 100 IU/kg twice daily for 2–8 days.
Note: It should be administered concurrently with oral aspirin (100 to 325 mg once daily). Treatment with Enoxaparin should be prescribed for a minimum of 2 days and continued until clinical stabilization.
Prevention of thrombus formation in the extracorporeal circulation during haemodialysis: The recommended dose of Enoxaparin is 100 IU/kg.
For patients with a high risk of hemorrhage, the dose should be reduced to 50 IU/kg for double vascular access or 75 IU/kg for single vascular access.
Note: During haemodialysis, Enoxaparin should be introduced into the arterial line of the circuit at the beginning of the dialysis session. The effect of the dose is usually sufficient for a 4-hour session. However, if fibrin rings are found, a further dose of 50 to 100 IU/kg may be given.
Prophylaxis of venous thromboembolic disease in surgical patients: Patients undergoing general surgery with a moderate thromboembolism risk (e.g., abdominal surgery): 2,000 IU or 4,000 IU once daily by subcutaneous injection for 7 to 10 days. The first injection should be given 2 hours prior to surgery.
Patients undergoing orthopedic surgery with a high thromboembolism risk: 4,000 IU once daily by subcutaneous injection for 7 to 10 days. The first injection should be given 12 hours prior to surgery.
Note: A longer treatment duration may be appropriate for some patients. Therapy with 4,000 IU once daily for 3 weeks following the initial treatment has been proven beneficial in orthopedic surgery.
Prophylaxis of venous thromboembolic disease in medical patients: The recommended dose is 4,000 IU once daily by subcutaneous injection for 6 to 14 days.
No dosage adjustment is required for patients with moderate (creatinine clearance 30–50 ml/min) or mild (creatinine clearance 50–80 ml/min) renal impairment. However, all such patients should be observed carefully for signs and symptoms of bleeding.
For patients with severe (creatinine clearance <30 ml/min) renal impairment:
Prophylactic dose: 2,000 IU once daily.
Therapeutic dose: 100 IU/kg once daily.
Elderly: No dosage adjustment is necessary unless kidney function is impaired.
Children: The safety and effectiveness of Enoxaparin in pediatric patients have not been established.

Common side effects include:
Hemorrhage (bleeding)
Thrombocytopenia
Elevations of serum aminotransferase
Pain and bluish marks at injection sites
Skin rash at injection sites
Cases of neuraxial hematomas have been reported with the concurrent use of Enoxaparin and spinal/epidural anesthesia or spinal puncture, resulting in varying degrees of neurological injuries.
Enoxaparin injection should not be administered via the intramuscular route.
Enoxaparin should be used with caution in conditions with an increased potential for bleeding, such as impaired hemostasis, a history of peptic ulcer, recent ischemic stroke, uncontrolled severe arterial hypertension, diabetic retinopathy, recent neuro-ophthalmologic surgery, and low-weight patients.
It is recommended that platelet counts be measured before initiating treatment and regularly thereafter.
Enoxaparin is contraindicated in patients with:
Hypersensitivity to Enoxaparin, heparin, or other low molecular weight heparins
Major clotting disorders such as a history of thrombocytopenia
Active gastrointestinal ulcers or organic lesions likely to bleed
Recent hemorrhagic vascular cerebral stroke
Although rare, cutaneous or systemic allergic reactions may occur.
Pregnancy:
Enoxaparin is classified as Pregnancy Category B. In humans, there is no evidence that Enoxaparin crosses the placental barrier.
It should be used during pregnancy only if the physician has established a clear need.
Enoxaparin is not recommended for pregnant women with prosthetic heart valves.
Lactation:
It is not known whether this drug is excreted in human milk.
Because many drugs are excreted in human milk, caution should be exercised when Enoxaparin is administered to nursing women.
Accidental overdosage of Enoxaparin may lead to hemorrhagic complications. Injected Enoxaparin may be largely neutralized by the slow intravenous (IV) injection of protamine sulfate (1% solution).
The dose of protamine sulfate should be equal to the dose of Enoxaparin injected: 1 mg of protamine sulfate should be administered to neutralize 1 mg of Enoxaparin.
Enoxaparin should be stored below 25°C.
Do not store in a refrigerator or freezer.