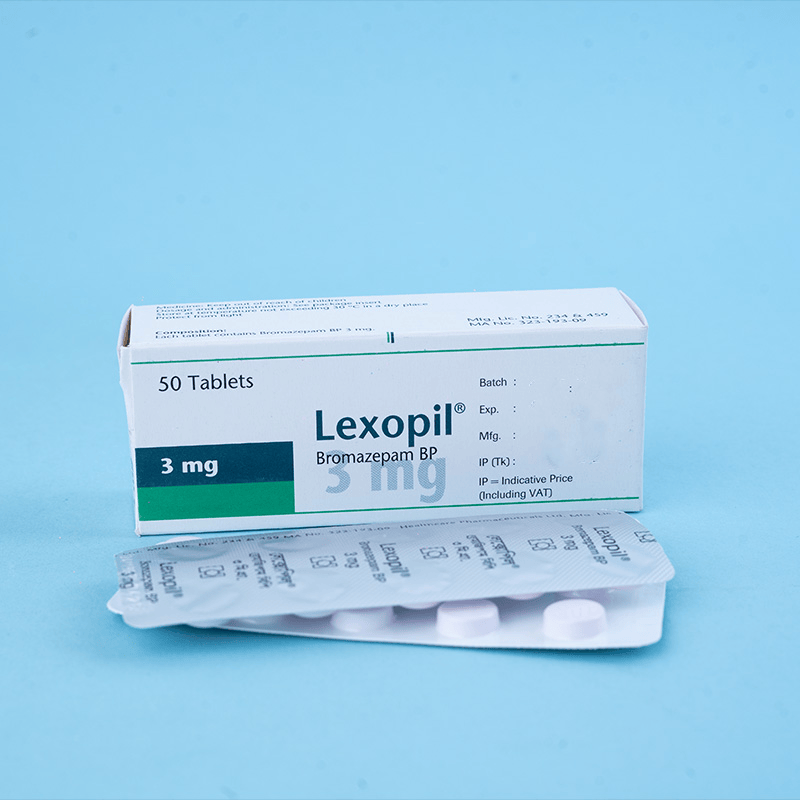
Lexopil® is a powerful psychotropic agent. In low doses, it selectively reduces tension and anxiety. In high doses, sedative and muscle-relaxing properties appear.
Emotional disturbances: Acute tension and anxiety states, difficulties in interpersonal contact, agitation, insomnia, anxious and agitated depressive reactions. Functional disturbances in the cardiovascular and respiratory systems: Pseudoangina pectoris, precordial anxiety, tachycardia, emotiogenic hypertension, dyspnea, and hyperventilation.In the gastrointestinal system: Irritable bowel syndrome, epigastric pain, spasms, bloating, diarrhea, etc.In the genitourinary system: Frequency, irritable bladder, and dysmenorrhea. Psychosomatic disorders: Psychogenic headache, psychogenic dermatosis, asthma, gastric and duodenal ulcers, ulcerative colitis. Emotional reactions to chronic organic disease. Adjuvant to psychotherapy in psychoneurosis.
Standard dosage: Outpatient therapy: 1.5–3 mg up to three times daily.
Severe cases (especially in hospital): 6–12 mg two or three times daily.
These amounts are general recommendations, and dosage should be individually determined. Treatment of outpatients should begin with low doses, gradually increasing to the optimum level. The duration of treatment should be as short as possible. Patients should be reassessed regularly, and the need for continued treatment should be evaluated, especially if the patient is symptom-free.
The overall treatment duration should generally not exceed 8–12 weeks, including a tapering-off process. In certain cases, an extension beyond this period may be necessary, but only after re-evaluation of the patient’s status by a specialist.
Special dosage instructions:
Lexopil® is usually not indicated for children. However, if a physician deems treatment necessary, the dose should be adjusted according to their low body weight (about 0.1–0.3 mg/kg body weight).
Elderly patients and those with impaired hepatic function require lower doses due to individual variations in sensitivity and pharmacokinetics.
Lexopil® must not be administered to patients with known hypersensitivity to benzodiazepines, severe respiratory insufficiency, or severe hepatic insufficiency (benzodiazepines are contraindicated for patients with severe hepatic insufficiency as they may cause encephalopathy), or sleep apnea syndrome.
Dependence: The use of benzodiazepines and benzodiazepine-like agents may lead to the development of physical and psychological dependence. The risk increases with dose and treatment duration and is hiher in predisposed patients with a history of alcohol or drug abuse.
Withdrawal: Once physical dependence has developed, discontinuation of treatment may be accompanied by withdrawal symptoms, including headaches, muscle pain, extreme anxiety, tension, restlessness, confusion, and irritability. In severe cases, symptoms such as derealization, depersonalization, hyperacusis, numbness and tingling of extremities, hypersensitivity to light, noise, and physical contact, hallucinations, or epileptic seizures may occur.
Rebound anxiety, a transient syndrome where the symptoms that led to treatment with Lexopil® return in an enhanced form, may occur upon withdrawal. It may be accompanied by mood changes, anxiety, sleep disturbances, and restlessness. To minimize these risks, dosage should be gradually reduced.
Amnesia: Benzodiazepines may cause anterograde amnesia, especially at higher therapeutic doses (documented at 6 mg), with the risk increasing at higher dosages.
Duration of treatment: Patients should be informed at the start of treatment that it will be of limited duration and be made aware of the possibility of rebound symptoms upon discontinuation.
Patients should be monitored regularly at the start of treatment to minimize dosage and prevent overdose due to accumulation.
Withdrawal symptoms may occur when switching to a benzodiazepine with a significantly shorter elimination half-life.
Benzodiazepines should not be used alone to treat depression or anxiety associated with depression (as they may precipitate suicide in such patients).
They are not recommended for the primary treatment of psychotic illnesses.
Patients with known or suspected dependence on alcohol, medications, or drugs should not take benzodiazepines except in rare situations under strict medical supervision.

Patients with myasthenia gravis require caution due to pre-existing muscle weakness.
Patients with chronic respiratory insufficiency should be monitored due to the risk of respiratory depression.
Effects on driving and machinery operation:
Sedation, amnesia, and impaired muscular function may adversely affect the ability to drive or use machinery, with increased risk if alcohol has been consumed.
The safety of bromazepam use during pregnancy has not been established. Some studies suggest an increased risk of congenital malformations associated with the use of minor tranquilizers (diazepam, meprobamate, and chlordiazepoxide) during the first trimester.
Bromazepam should be avoided during pregnancy unless no safer alternative is available. If prescribed to a woman of childbearing potential, she should be advised to consult her physician if she plans to become pregnant or suspects she is pregnant.
Administration of bromazepam during the last three months of pregnancy or labor should only occur under strict medical indication, as it may cause hypothermia, hypotonia, and moderate respiratory depression in the neonate.
Infants born to mothers who used benzodiazepines chronically during late pregnancy may develop physical dependence and risk withdrawal symptoms postnatally.
Since benzodiazepines pass into breast milk, nursing mothers should not take Lexopil®.
Lexopil® is well tolerated at therapeutic doses. Possible side effects include:
Common effects: Fatigue, drowsiness, muscle weakness, numbed emotions, reduced alertness, confusion, headache, dizziness, ataxia, or double vision. These usually occur at the start of therapy and diminish with continued use.
Occasional effects: Gastrointestinal disturbances, changes in libido, and skin reactions.
Amnesia: Anterograde amnesia may occur, with higher risk at increased doses, sometimes associated with inappropriate behavior.
Pre-existing depression: Symptoms may be unmasked during benzodiazepine use.
Paradoxical reactions: Restlessness, agitation, irritability, aggressiveness, delusions, rage, nightmares, hallucinations, psychosis, inappropriate behavior, or other adverse behavioral effects. These reactions are more likely in children and the elderly and warrant discontinuation of treatment.
Dependence and withdrawal: Chronic use (even at therapeutic doses) may lead to physical dependence, with withdrawal or rebound symptoms upon discontinuation. Psychological dependence and benzodiazepine abuse have been reported.
Alcohol: May intensify Lexopil®’s effects. Concomitant use should be avoided.
Centrally active drugs: Lexopil®’s sedative effect may be enhanced when combined with antidepressants, hypnotics, narcotic analgesics, antipsychotics, anxiolytics/sedatives, antiepileptics, sedative antihistamines, or anesthetics.
Narcotic analgesics: Increased euphoria may occur, leading to heightened psychological dependence.
Enzyme inhibitors: Certain compounds that inhibit hepatic enzymes may affect the metabolism of benzodiazepines. Co-administration of cimetidine may prolong the elimination half-life of bromazepam.
Each box contains 5 × 10 tablets in a blister pack.
Store in a cool, dry place below 30°C. Protect from light.
Medicine: Keep out of reach of children.