Rivaroxaban is a selective inhibitor of Factor Xa. It does not require a cofactor (such as Antithrombin III) for activity. Rivaroxaban inhibits free Factor Xa and prothrombinase activity. It has no direct effect on platelet aggregation but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting Factor Xa, Rivaroxaban decreases thrombin generation.
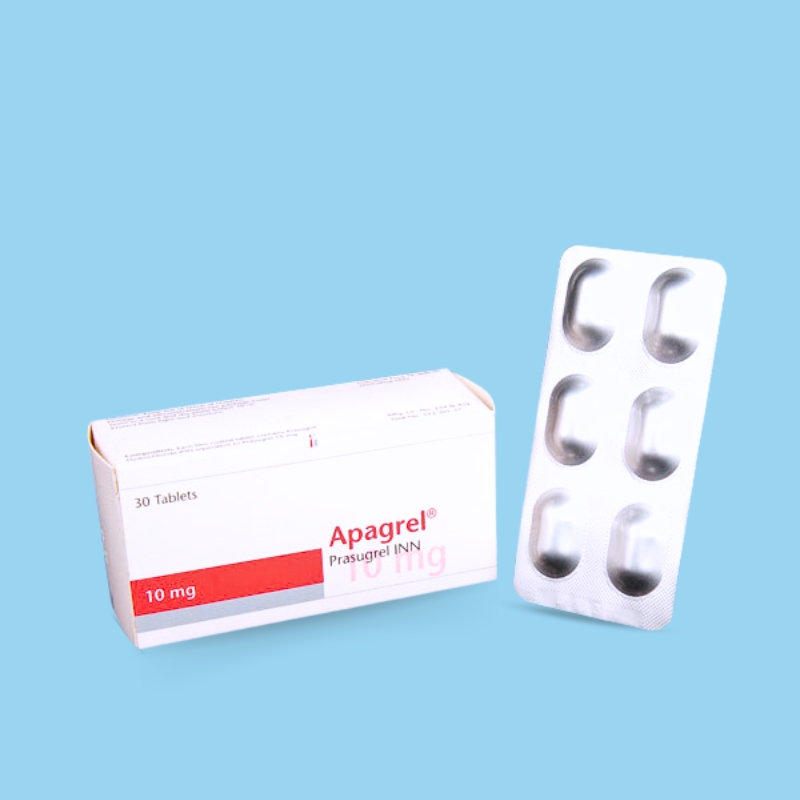
Rivaroxaban is indicated for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation; the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE); reducing the risk of recurrence of DVT and/or PE in patients at continued risk after completion of initial treatment lasting at least six months; prophylaxis of DVT, which may lead to PE, in patients undergoing knee or hip replacement surgery; and reducing the risk of major cardiovascular events (cardiovascular death, myocardial infarction [MI], and stroke) in patients with chronic coronary artery disease (CAD) or peripheral artery disease (PAD).
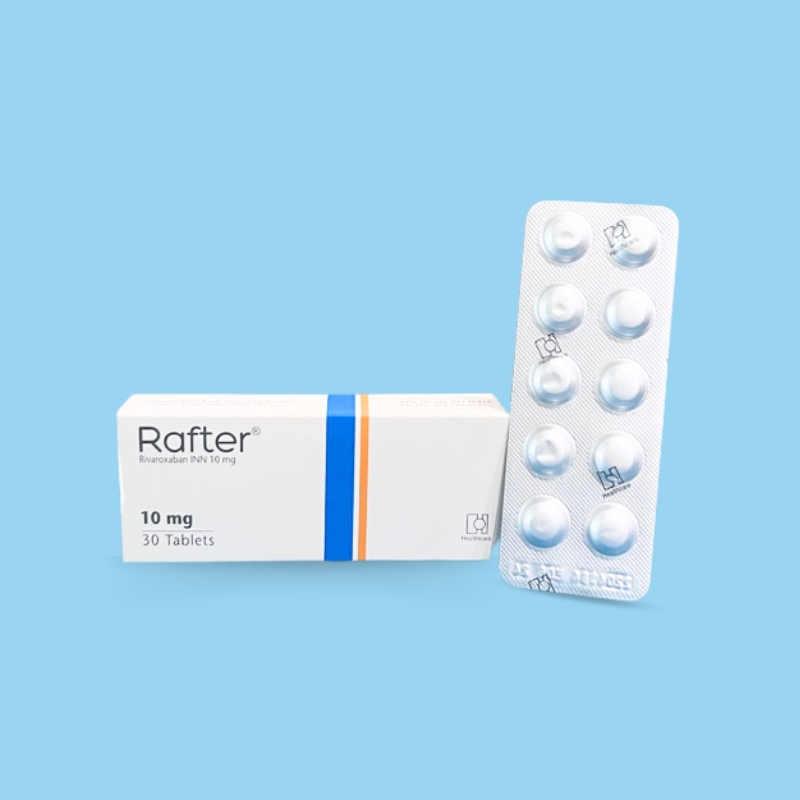
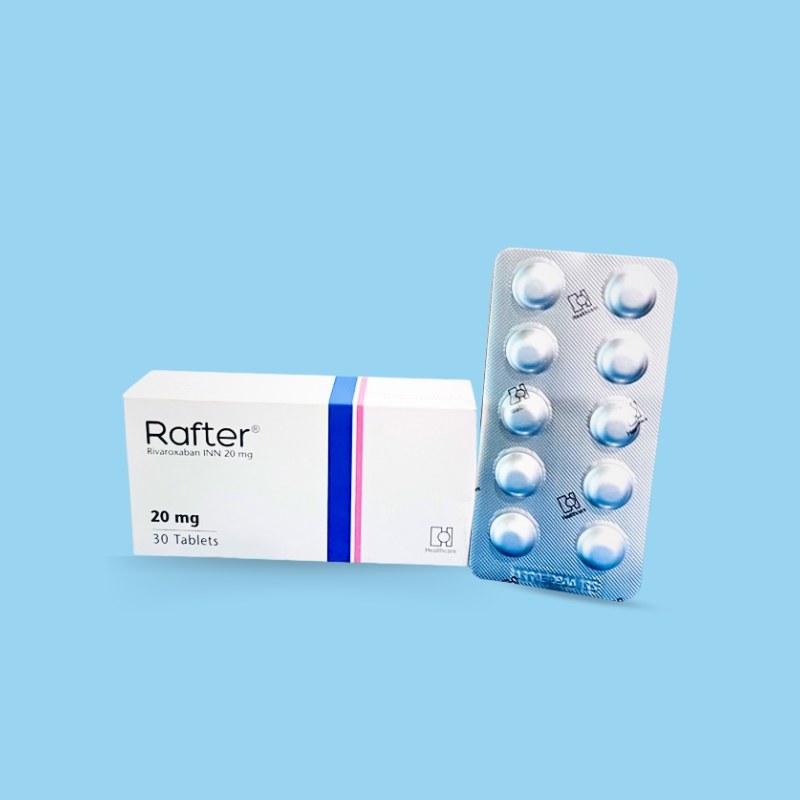
Nonvalvular Atrial Fibrillation: 15 or 20 mg once daily with food.
Treatment of DVT and/or PE: 15 mg orally twice daily with food for the first 21 days, followed by 20 mg orally once daily with food for the remaining treatment.
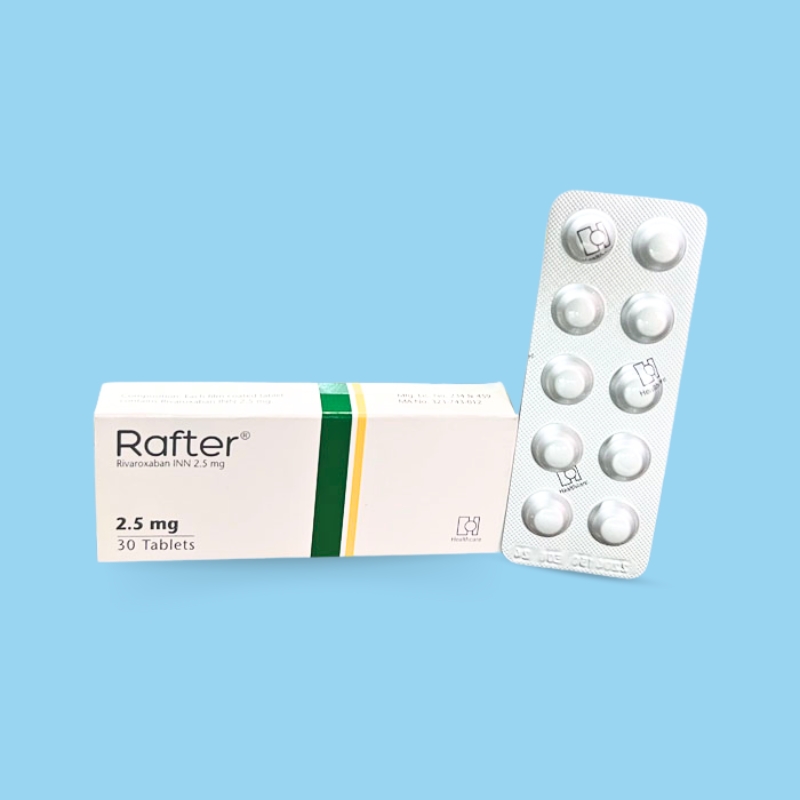
Reduction in the Risk of Recurrence of DVT and/or PE in Patients at Continued Risk: 10 mg once daily with or without food, after at least six months of standard anticoagulant treatment.
Prophylaxis of DVT Following Hip or Knee Replacement Surgery: 10 mg orally once daily with or without food.
Prophylaxis of VTE in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding: 10 mg once daily, with or without food, in hospital and after hospital discharge for a total recommended duration of 31 to 39 days.
CAD or PAD: 2.5 mg orally twice daily with or without food, in combination with aspirin (75-100 mg) once daily.
Active pathological bleeding and severe hypersensitivity reactions.
Risk of Bleeding: Rivaroxaban can cause serious and fatal bleeding. An agent to reverse the activity of Rivaroxaban is available.
Pregnancy-Related Hemorrhage: Use Rivaroxaban with caution in pregnant women due to the potential for obstetric hemorrhage and/or emergent delivery.
Prosthetic Heart Valves: Rivaroxaban use is not recommended in patients with prosthetic heart valves.
Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome: Rivaroxaban use is not recommended.
Hypersensitivity
Bleeding
Increased Risk of Stroke After Discontinuation in Nonvalvular Atrial Fibrillation
Bleeding Risk
Spinal/Epidural Hematoma
Other Adverse Reactions
Non-hemorrhagic adverse reactions reported in ≥1% of rivaroxaban-treated patients in the EINSTEIN DVT and EINSTEIN PE studies include gastrointestinal disorders, fatigue, back pain, muscle spasm, dizziness, anxiety, depression, and insomnia.
Avoid combined P-gp and strong CYP3A inhibitors and inducers. Avoid concomitant use of anticoagulants.
Renal Impairment: Avoid or adjust the dose.
Hepatic Impairment: Avoid use in patients with Child-Pugh B and C hepatic impairment or hepatic disease associated with coagulopathy.

The limited available data on Rivaroxaban in pregnant women are insufficient to determine a drug-associated risk of adverse developmental outcomes. Use Rivaroxaban with caution in pregnant patients because of the potential for pregnancy-related hemorrhage and/or emergent delivery. The anticoagulant effect of Rivaroxaban cannot be reliably monitored with standard laboratory testing. Consider the benefits and risks for the mother and possible risks to the fetus when prescribing Rivaroxaban to a pregnant woman.

Rivaroxaban has been detected in human milk. There are insufficient data to determine the effects of Rivaroxaban on the breastfed child or milk production. Rivaroxaban and/or its metabolites were present in the milk of rats. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Rivaroxaban and any potential adverse effects on the breastfed infant.
Safety and effectiveness in pediatric patients have not been established.
Of the total number of patients in the RECORD 1-3 clinical studies evaluating Rivaroxaban, about 54% were 65 years and older, while about 15% were over 75 years. In ROCKET AF, approximately 77% were 65 years and older, and about 38% were over 75 years. In the EINSTEIN DVT, PE, and Extension clinical studies, approximately 37% were 65 years and older, and about 16% were over 75 years. In EINSTEIN CHOICE, approximately 39% were 65 years and older, and about 12% were over 75 years. In the MAGELLAN study, approximately 67% were 65 years and older, and about 37% were over 75 years. In the COMPASS study, approximately 76% were 65 years and older, and about 17% were over 75 years. In clinical trials, the efficacy of Rivaroxaban in the elderly (65 years or older) was similar to that seen in patients younger than 65 years. Both thrombotic and bleeding event rates were higher in these older patients.
Of the total number of patients in the RECORD 1-3 clinical studies evaluating Rivaroxaban, about 54% were 65 years and older, while about 15% were over 75 years. In ROCKET AF, approximately 77% were 65 years and older, and about 38% were over 75 years. In the EINSTEIN DVT, PE, and Extension clinical studies, approximately 37% were 65 years and older, and about 16% were over 75 years. In EINSTEIN CHOICE, approximately 39% were 65 years and older, and about 12% were over 75 years. In the MAGELLAN study, approximately 67% were 65 years and older, and about 37% were over 75 years. In the COMPASS study, approximately 76% were 65 years and older, and about 17% were over 75 years. In clinical trials, the efficacy of Rivaroxaban in the elderly (65 years or older) was similar to that seen in patients younger than 65 years. Both thrombotic and bleeding event rates were higher in these older patients.
Store at a temperature not exceeding 30°C in a dry place. Protect from light and moisture.
Medicine: Keep out of reach of children.