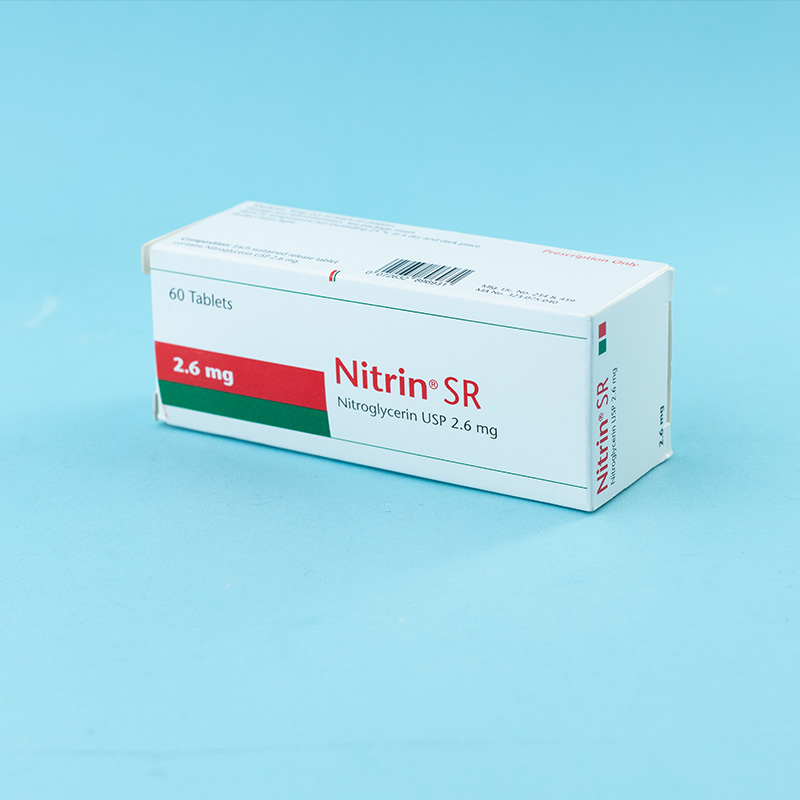
Nitrin® SR is a potent coronary vasodilator. It also reduces venous return and, thus, left ventricular workload. Nitrin® SR possesses sustained effects because its absorption from the gastrointestinal tract is prolonged and continuous due to the sustained release of the active substance from the formulation.
Prevention and treatment of effort-induced and vasospastic angina. It is also used as a supplementary treatment for heart failure that is refractory to digitalis and diuretic therapy.
Adults and elderly patients: Dosage should be tailored to the requirements of the individual patient, depending on the severity of the illness and the occurrence of side effects, but it is usually one or two tablets/capsules taken two to three times daily before meals.
Children: Not recommended.
Administration: Nitrin® SR is for oral use only. The tablet/capsule must be swallowed whole and not chewed.
Nitroglycerin USP should not be used in patients with severe anemia, head trauma, cerebral hemorrhage, or incipient glaucoma. Sildenafil has been shown to potentiate the hypotensive effects of nitrates; therefore, its co-administration with nitrates or nitric oxide donors is strictly contraindicated.
As with other drugs used to treat angina pectoris, abrupt discontinuation of therapy may lead to a worsening of symptoms. When discontinuing long-term treatment, the dosage should be reduced gradually over several days.
Nitrin® SR may enhance the effects of peripheral vasodilators. The hypotensive effects of nitrates are further increased when taken concurrently with sildenafil.
There is no conclusive evidence regarding the safety of nitrates during pregnancy and lactation. Nitrin® SR should only be administered to pregnant women and nursing mothers if deemed essential by a physician.
Headache, dizziness, and mild gastrointestinal symptoms may occur. These side effects typically subside upon dosage reduction and do not usually require discontinuation of treatment.
In the event of accidental or deliberate overdose, toxic effects of Nitroglycerin may include vomiting, restlessness, cyanosis, methemoglobinemia, syncope, and tachycardia. Gastric aspiration and lavage should be performed, and patients should receive appropriate respiratory and circulatory support.
Following oral administration, Nitroglycerin is rapidly metabolized to glyceryl-1,2-dinitrate and glyceryl-1,3-dinitrate. Although these metabolites are less potent, they likely provide the predominant pharmacological effect. Studies with Nitroglycerin 2.6 mg demonstrate a tmax for both metabolites of approximately 1 hour and an apparent t1/2 of approximately 2 hours. There is evidence of activity lasting for six hours or more.
Store at a temperature not exceeding 25°C and keep away from light.
Medicine: Keep out of reach of children.