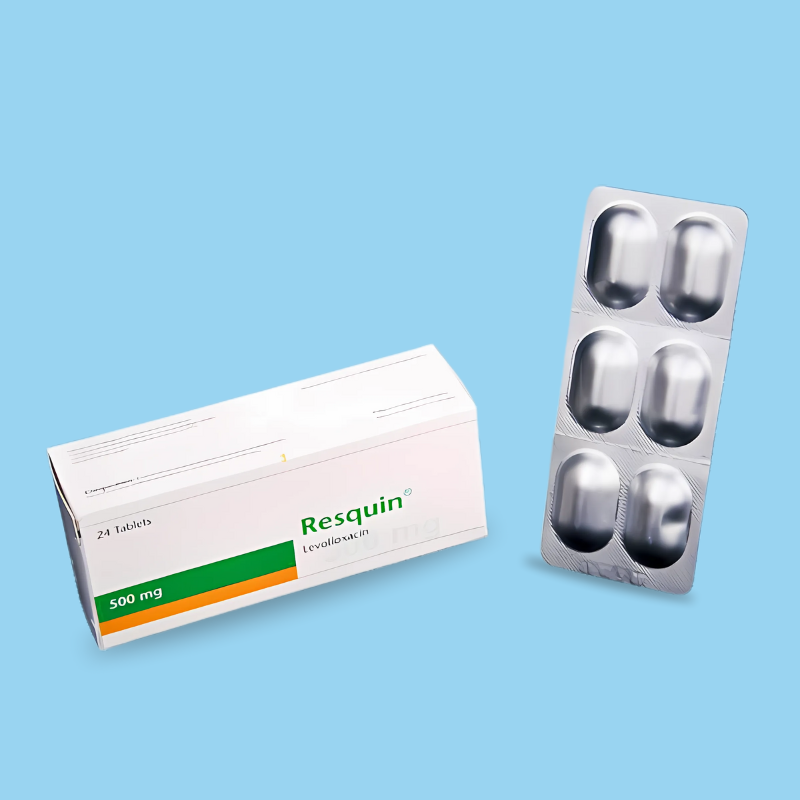
Rocipro®, containing ciprofloxacin, is a synthetic quinolone anti-infective agent. Ciprofloxacin has a broad spectrum of activity. It is active against most gram-negative aerobic bacteria, including Enterobacteriaceae and Pseudomonas aeruginosa. Ciprofloxacin is also active against gram-positive aerobic bacteria, including penicillinase-producing, non-penicillinase-producing, and methicillin-resistant staphylococci, although many strains of streptococci are relatively resistant to the drug. The bactericidal action of ciprofloxacin results from interference with the enzyme DNA gyrase, which is needed for the synthesis of bacterial DNA.
Following oral administration, it is rapidly and well absorbed from the gastrointestinal tract. It is widely distributed into body tissues and fluids. The half-life is about 3.5 hours. About 30% to 50% of an oral dose of ciprofloxacin is excreted in the urine within 24 hours as an unchanged drug and biologically active metabolites.

Ciprofloxacin is indicated for the treatment of single or mixed infections caused by two or more susceptible organisms. It can also be used for infections caused by organisms resistant to other antibiotics, including aminoglycosides, penicillins, and cephalosporins.
As antibacterial concentrations of ciprofloxacin are obtained in serum and body tissues as well as in urine following oral administration, ciprofloxacin has been suggested for use in treating a wide range of infections caused by susceptible organisms. These include infections of the urinary, respiratory, and gastrointestinal tracts, gonorrhea, and septicemia. The extensive tissue penetration of ciprofloxacin, combined with its enhanced antibacterial activity (including antipseudomonal activity), enables it to be used alone (pending sensitivity results) or in combination with an aminoglycoside or beta-lactam antibiotics, for instance, when severe neutropenia is present. It may also be used with an antibiotic active against anaerobes where Bacteroides fragilis is suspected.
Rocipro® is indicated for the treatment of the following infections caused by sensitive bacteria:
Severe systemic infections: e.g., septicemia, bacteremia, peritonitis, infections in immunosuppressed patients with hematological or solid tumors, and infections in intensive care unit patients with specific problems such as infected burns.
Respiratory tract infections: lobar and bronchopneumonia, acute and chronic bronchitis, acute exacerbation of cystic fibrosis, bronchiectasis, and empyema.
Urinary tract infections: uncomplicated and complicated urethritis, cystitis, pyelonephritis, prostatitis, and epididymitis.
Skin and soft tissue infections: e.g., infected ulcers, wound infections, abscesses, cellulitis, otitis externa, erysipelas, and infected burns.
Gastrointestinal infections: e.g., enteric fever and infective diarrhea.
Infections of the biliary tract: e.g., cholangitis, cholecystitis, and empyema of the gall bladder.
Intra-abdominal infections: e.g., peritonitis and intra-abdominal abscesses.
Bone and joint infections: osteomyelitis and septic arthritis.
Pelvic infections: e.g., salpingitis, endometritis, and pelvic inflammatory diseases.
Eye, ear, nose, and throat infections: e.g., otitis media, sinusitis, mastoiditis, and tonsillitis.
Gonorrhea: including urethral, rectal, and pharyngeal gonorrhea caused by beta-lactamase-producing organisms or organisms moderately sensitive to penicillin.
General dosage recommendations: The dosage of ciprofloxacin is determined by the severity and type of infection, the sensitivity of the causative organism(s), and the age, weight, and renal function of the patient.
Adults: The dosage range for adults is 100–750 mg twice daily.
In infections of the lower and upper urinary tract (depending on severity): 250–500 mg twice daily.
In respiratory tract infections: 250–500 mg twice daily for both upper and lower respiratory tract infections, depending on severity. For the treatment of known Streptococcus pneumoniae infection, the recommended dosage is 750 mg twice daily.
In gonorrhea: A single dose of 250 or 500 mg.
In most other infections, 500–750 mg twice daily should be administered.
Cystic fibrosis: In adults with pseudomonal infections of the lower respiratory tract, the normal dose is 750 mg twice daily.
As the pharmacokinetics of ciprofloxacin remain unchanged in patients with cystic fibrosis, the low body weight of these patients should be considered when determining dosage.
Impaired renal function: Dosage adjustment is usually not required except in patients with severe renal impairment (serum creatinine > 265 µmol/L or creatinine clearance < 20 mL/min). If adjustment is necessary, this may be achieved by reducing the total daily dose by half, although monitoring of drug serum levels provides the most reliable basis for dose adjustment.
Elderly: Although higher ciprofloxacin serum levels are found in the elderly, no dosage adjustment is necessary.
Adolescents and children: As with other drugs in this class, ciprofloxacin has been shown to cause arthropathy in weight-bearing joints of immature animals. Although the relevance to humans is unknown, its use in children, growing children, and adolescents is not recommended. However, where the benefits outweigh the potential risk, the dosage should be 7.5–15 mg/kg/day, depending on infection severity, administered in two divided doses.
Duration of treatment: The duration of treatment depends on infection severity, clinical response, and bacteriological findings. For acute infections, the usual treatment period is 5 to 10 days with Rocipro® tablets. Generally, treatment should continue for three days after symptoms have disappeared.
Use in Pregnancy and Lactation
Reproduction studies in mice, rats, and rabbits using parenteral and oral administration did not show teratogenicity, impairment of fertility, or peri/postnatal development issues. However, as with other quinolones, ciprofloxacin has been shown to cause arthropathy in immature animals. Therefore, its use during pregnancy is not recommended. Studies in rats have indicated that ciprofloxacin is secreted in breast milk, so its administration to nursing mothers is not recommended.
Ciprofloxacin should be used with caution in patients with suspected or known CNS disorders such as arteriosclerosis, epilepsy, or other factors that predispose them to seizures and convulsions.
Ciprofloxacin may be taken with or without meals, and fluids should be consumed liberally.
Ciprofloxacin should not be taken concurrently with magnesium/aluminum antacids, sucralfate, or other products containing calcium, iron, and zinc. These products may be taken two hours after or six hours before ciprofloxacin.
Ciprofloxacin should not be taken with milk or yogurt alone, as absorption may be significantly reduced. However, dietary calcium as part of a meal does not significantly affect absorption.
Ciprofloxacin is contraindicated in patients with a history of hypersensitivity to ciprofloxacin or other quinolones
Adverse effects include nausea, gastrointestinal disturbances, headache, dizziness, and skin rashes. Crystalluria has been reported with high doses.
Store in a cool, dry place below 30°C. Protect from light.
Keep out of reach of children.

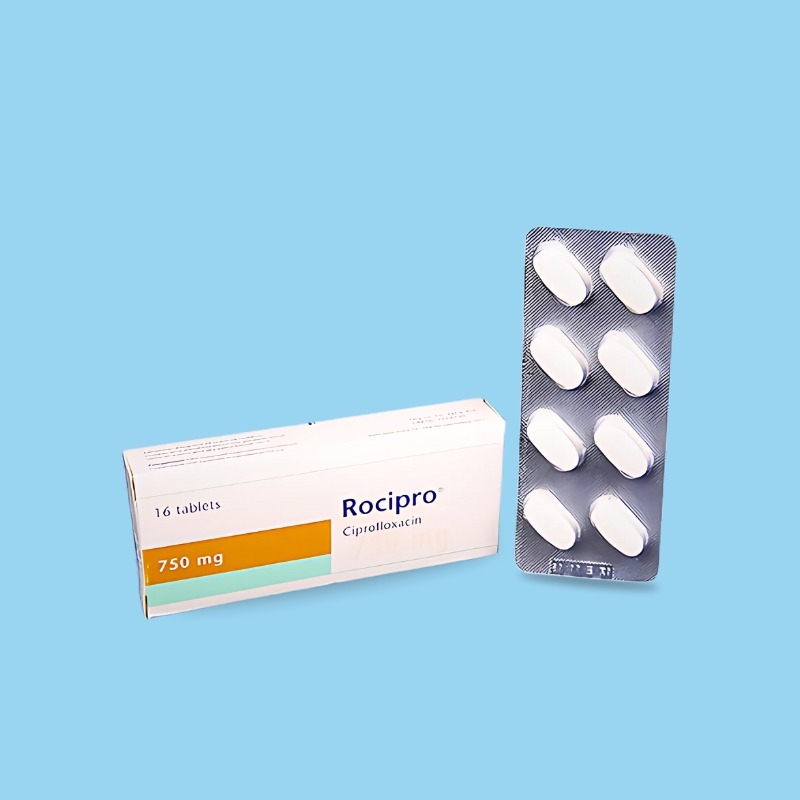
.jpg)