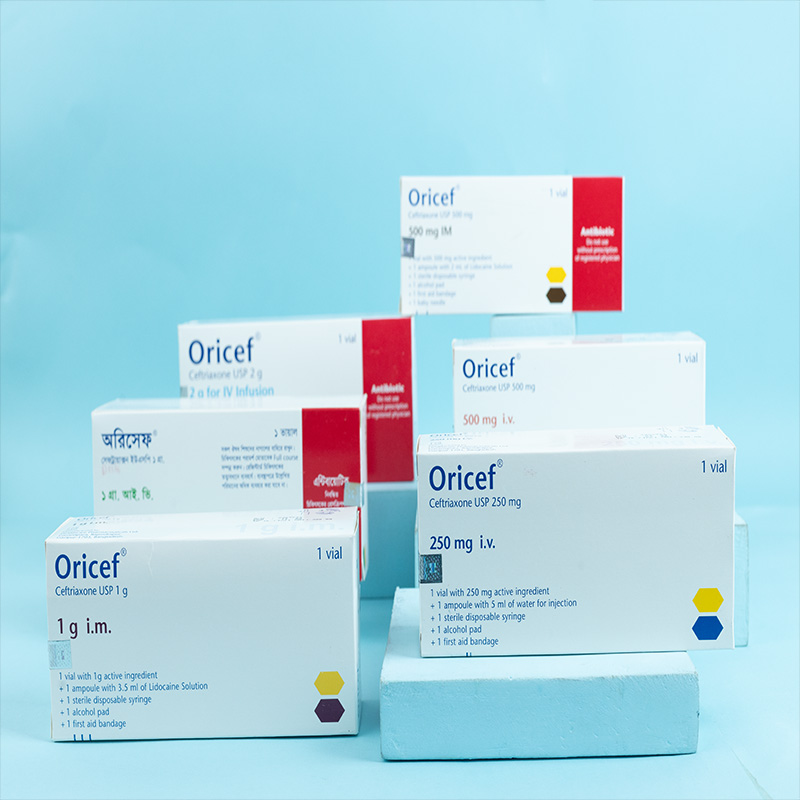
250 mg i.v./i.m. Injection: Each vial contains Ceftriaxone Sodium USP 298.23 mg, equivalent to Ceftriaxone 250 mg.
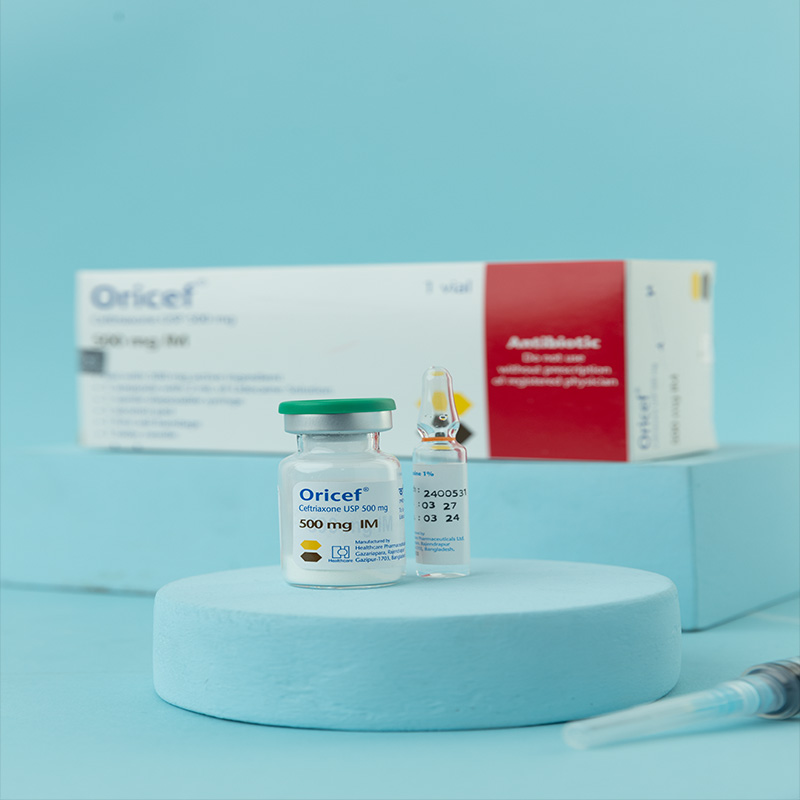
500 mg i.v./i.m. Injection: Each vial contains Ceftriaxone Sodium USP 596.46 mg, equivalent to Ceftriaxone 500 mg.
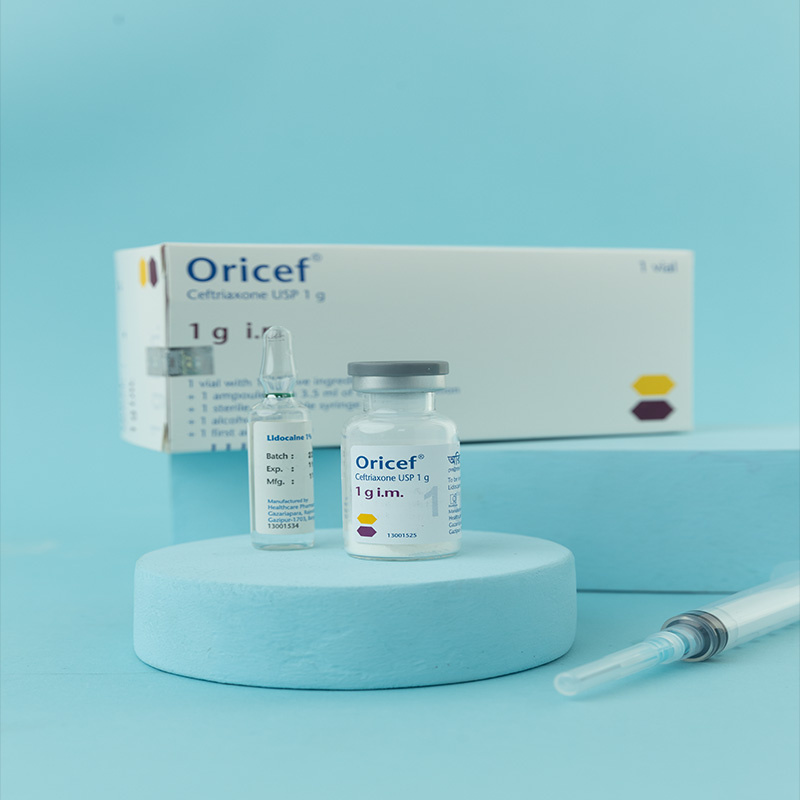
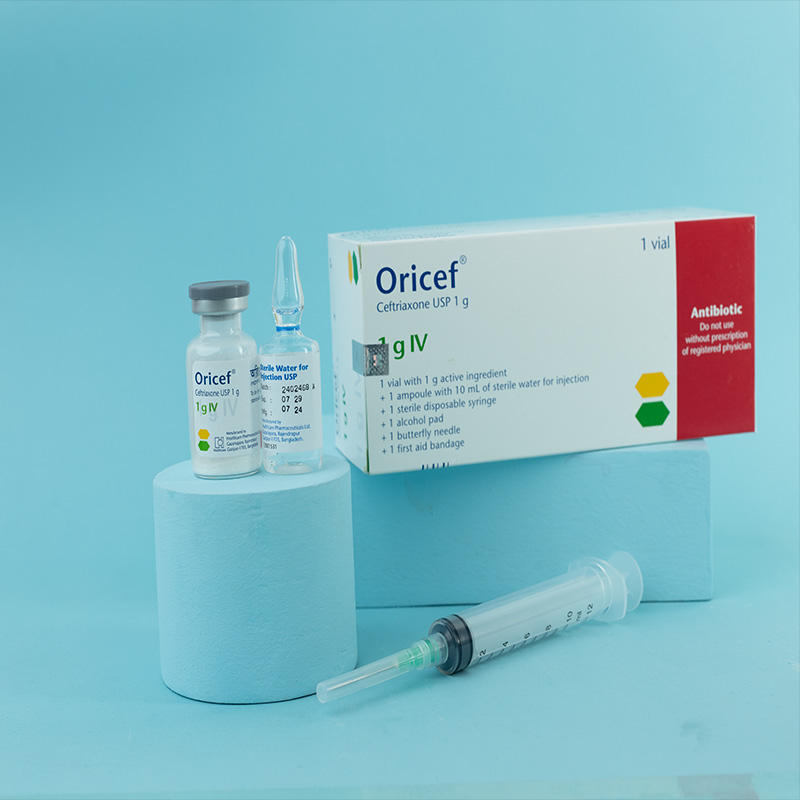
1 g i.v./i.m. Injection: Each vial contains Ceftriaxone Sodium USP 1.193 g, equivalent to Ceftriaxone 1 g.
2 g i.v. Infusion: Each vial contains Ceftriaxone Sodium USP 2.386 g, equivalent to Ceftriaxone 2 g.
Solvent for Parenteral Use:
For i.m. injection: 1% lidocaine hydrochloride solution.
For i.v. injection: Sterile water for injection.

The bactericidal activity of ceftriaxone results from its inhibition of bacterial cell wall synthesis. Ceftriaxone demonstrates in-vitro activity against a broad range of gram-positive and gram-negative microorganisms. It is highly stable against most β-lactamases, including penicillinases and cephalosporinases.
Microorganisms Susceptible to Ceftriaxone:
Gram-positive Aerobes:
Staphylococcus aureus (including penicillinase-producing strains)
Staphylococcus epidermidis
Streptococcus pneumoniae
Streptococcus Group A (Str. pyogenes), Group B (Str. agalactiae), Group C, Group G
Streptococcus viridans
Streptococcus bovis
Gram-negative Aerobes:
Aeromonas spp., Alcaligenes spp., Branhamella catarrhalis (β-lactamase positive/negative)
Citrobacter spp., Enterobacter spp. (some strains resistant), Escherichia coli
Haemophilus ducreyi, Haemophilus influenzae, Klebsiella spp., Moraxella spp.
Morganella morganii, Neisseria gonorrhoeae, Neisseria meningitidis, Proteus mirabilis
Pseudomonas aeruginosa (some strains resistant), Salmonella spp., Serratia spp., Shigella spp., Vibrio spp., Yersinia spp.
Anaerobic Organisms:
Bacteroides spp., Clostridium spp. (except Cl. difficile), Fusobacterium spp.
Peptococcus spp., Peptostreptococcus spp.
Pharmacokinetics
Absorption: Following a single i.m. dose of 1 g, the maximum plasma concentration (~81 mg/L) is achieved within 2–3 hours. Bioavailability is 100% for intramuscular administration.
Distribution: Ceftriaxone distributes extensively (7–12 L) and reaches effective concentrations in various tissues and fluids, including cerebrospinal, pleural, prostatic, and synovial fluids.

About 50–60% is excreted unchanged in the urine, and 40–50% via bile.
The elimination half-life is approximately 8 hours in adults.
Indications and Usage
Ceftriaxone is indicated for infections caused by susceptible microorganisms, including:
Sepsis, meningitis, abdominal infections, respiratory tract infections, bone and joint infections, urinary tract infections, and gonorrhea.
Dosage and Administration
Route: Parenteral
Adults/Children (≥12 years): 1–2 g once daily. In severe cases, doses up to 4 g/day may be required.
Neonates/Infants: 20–50 mg/kg daily for neonates; 20–80 mg/kg for older children.
Meningitis: 100 mg/kg once daily (maximum 4 g/day).

i.m. Injection: Dissolve 250 mg or 500 mg in 2 mL, and 1 g in 3.5 mL, of 1% lidocaine solution.
i.v. Injection: Dissolve 250 mg or 500 mg in 5 mL, and 1 g in 10 mL, of sterile water. Administer over 2–4 minutes.
Warnings and Precautions
Monitor for anaphylactic reactions.
Exercise caution in hyperbilirubinemic neonates.
Regularly monitor blood counts during prolonged therapy.

Monitor for anaphylactic reactions.
Exercise caution in hyperbilirubinemic neonates.
Regularly monitor blood counts during prolonged therapy.

Known hypersensitivity to cephalosporins.