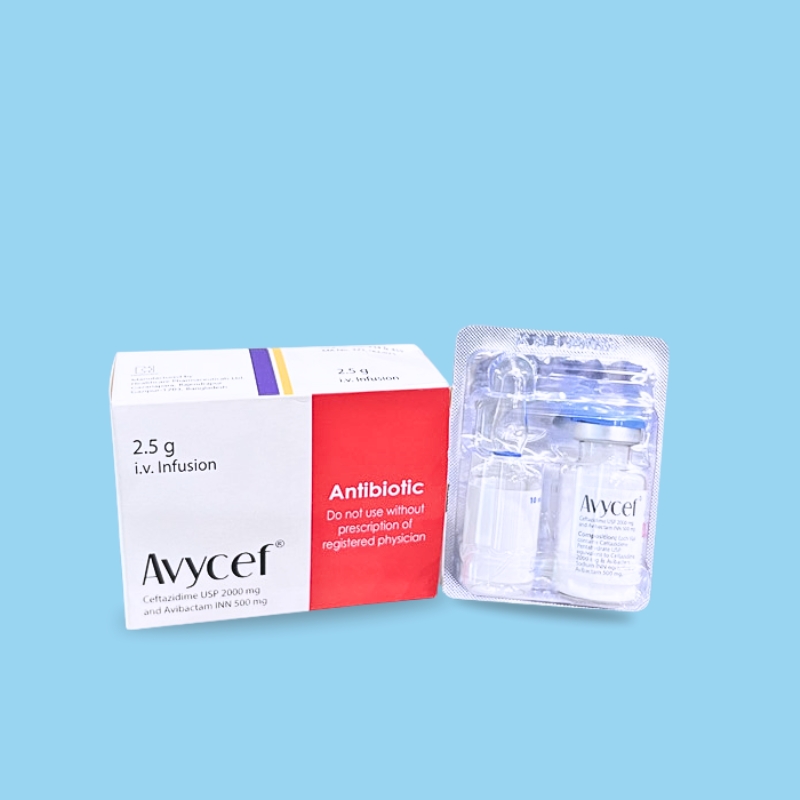
The ceftazidime component of Avycef® is a cephalosporin antibacterial drug with in vitro activity against certain Gram-negative and Gram-positive bacteria. The bactericidal action of ceftazidime is mediated through its binding to essential penicillin-binding proteins (PBPs). The avibactam component of Avycef® is a non-beta-lactam beta-lactamase inhibitor that inactivates certain beta-lactamases, which degrade ceftazidime. Avibactam does not reduce the activity of ceftazidime against ceftazidime-susceptible organisms.
Complicated intra-abdominal infections (cIAI)
Complicated urinary tract infections (cUTI), including pyelonephritis
Hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP)
Avycef® is supplied as a dry powder that must be constituted and subsequently diluted using an aseptic technique before intravenous infusion.
Reconstitution Directions
a) Constitute the powder in the Avycef® vial with 10 mL of one of the following solutions:
Sterile water for injection, USP
0.9% sodium chloride injection, USP (normal saline)
5% dextrose injection, USP
All combinations of dextrose injection and sodium chloride injection, USP, containing up to 2.5% dextrose, USP, and 0.45% sodium chloride, USP
Lactated Ringer’s injection, USP
b) Mix gently and ensure complete dissolution of the contents. The constituted Avycef® solution will have an approximate ceftazidime concentration of 167 mg/mL and an avibactam concentration of 42 mg/mL. The final volume is approximately 12 mL. The constituted solution is not for direct injection and must be diluted before intravenous infusion.
c) Prepare the required dose for intravenous infusion by withdrawing the appropriate volume, as determined by the following table, from the constituted vial.
d) Before infusion, further dilute the withdrawn volume of the constituted Avycef® solution with the same diluent used for constitution (except sterile water for injection) to achieve a ceftazidime concentration of 8 to 40 mg/mL and an avibactam concentration of 2 to 10 mg/mL in an infusion bag. If sterile water for injection was used for constitution, use one of the other approved diluents for dilution.
e) Mix gently and ensure complete dissolution of the contents. Visually inspect the diluted Avycef® solution for particulate matter and discoloration before administration. The color of the Avycef® infusion solution ranges from clear to light yellow.
f) Use the diluted Avycef® solution within 12 hours when stored at room temperature.
g) The diluted Avycef® solution may be stored under refrigeration (2 to 8°C) for up to 24 hours after dilution, followed by an additional 12-hour window for use at room temperature.
In the event of an overdose, discontinue Avycef® and provide general supportive treatment. Ceftazidime and avibactam can be removed by hemodialysis.
Avycef® is contraindicated in patients with known serious hypersensitivity to ceftazidime, avibactam, other avibactam-containing products, or other cephalosporin-class antibiotics.
Decreased efficacy in adult cIAI patients with baseline CrCl of 30 to ≤ 50 mL/min: Monitor CrCl at least daily in adult and pediatric patients with fluctuating renal function and adjust the Avycef® dosage accordingly.
Hypersensitivity reactions: Includes anaphylaxis and serious skin reactions. Cross-hypersensitivity may occur in patients with a history of penicillin allergy. If an allergic reaction occurs, discontinue Avycef® immediately.
Clostridium difficile-associated diarrhea (CDAD): CDAD has been reported with nearly all systemic antibacterial agents, including Avycef®. Evaluate the patient if diarrhea occurs.
Central nervous system reactions: Seizures and other neurological events may occur, particularly in patients with renal impairment. Dose adjustment is required for patients with renal dysfunction.
Adult cIAI, cUTI, and HABP/VABP Patients:
The most common adverse reactions in cIAI patients (≥ 5%, when used with metronidazole) are diarrhea, nausea, and vomiting.
The most common adverse reactions in cUTI patients (≥ 3%) are diarrhea and nausea.
The most common adverse reactions in HABP/VABP patients (≥ 5%) are diarrhea and vomiting.
Pediatric cIAI and cUTI Patients: The most common adverse reactions (> 3%) in pediatric patients are vomiting, diarrhea, rash, and infusion site phlebitis.
No clinical interaction study has been conducted on Avycef® or avibactam alone with probenecid. Co-administration of Avycef® with probenecid is not recommended.
Pregnancy: No adequate and well-controlled studies have been conducted on Avycef®, ceftazidime, or avibactam in pregnant women.
Lactation: No data are available on the effects of ceftazidime and avibactam on breastfed infants or milk production.
Store at a temperature not exceeding 25°C in a dry place. Protect from light and moisture.
Keep out of reach of children