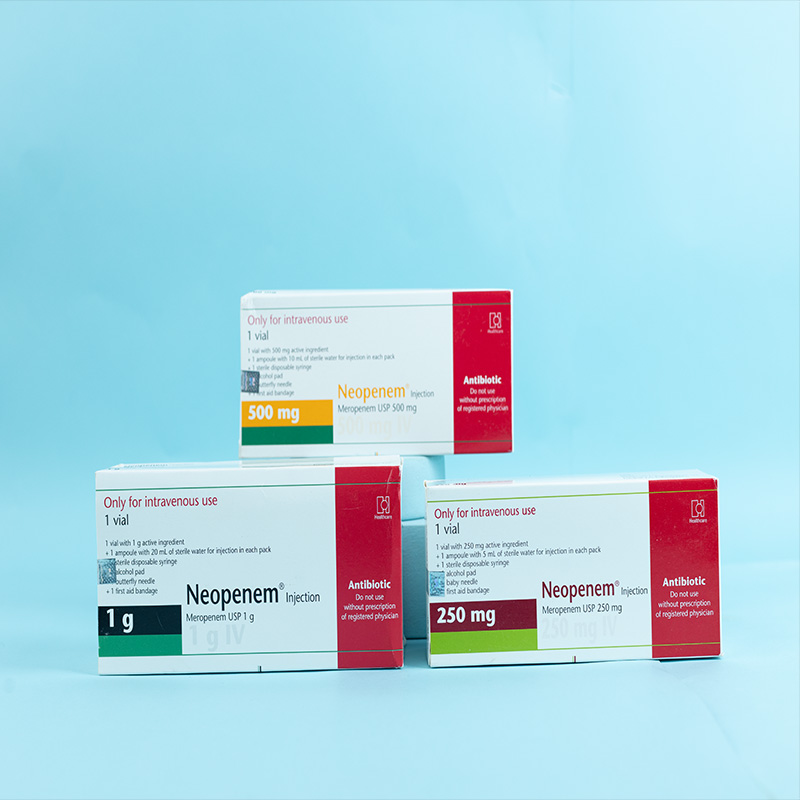
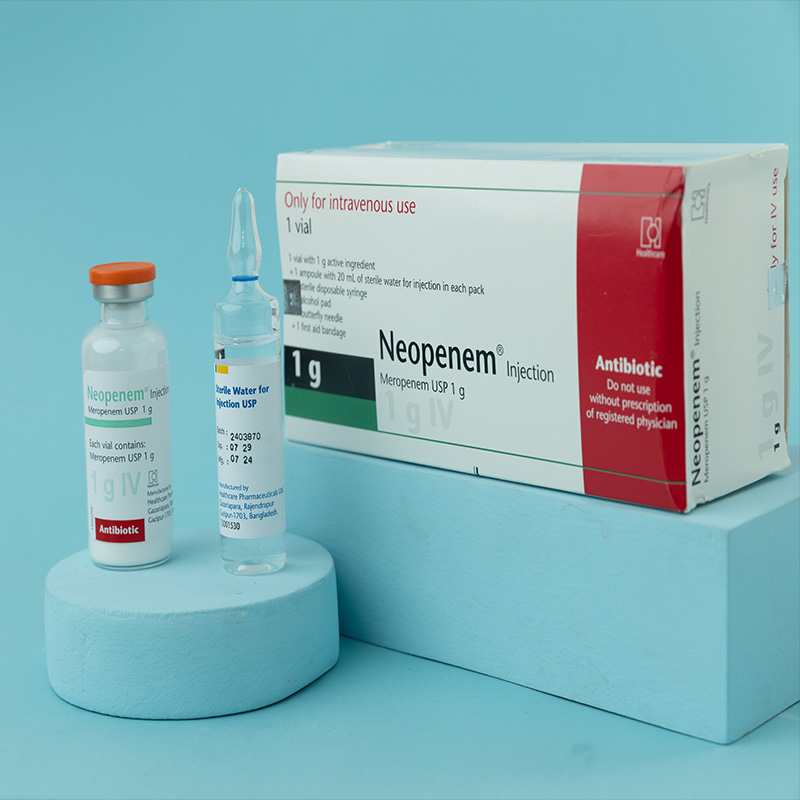
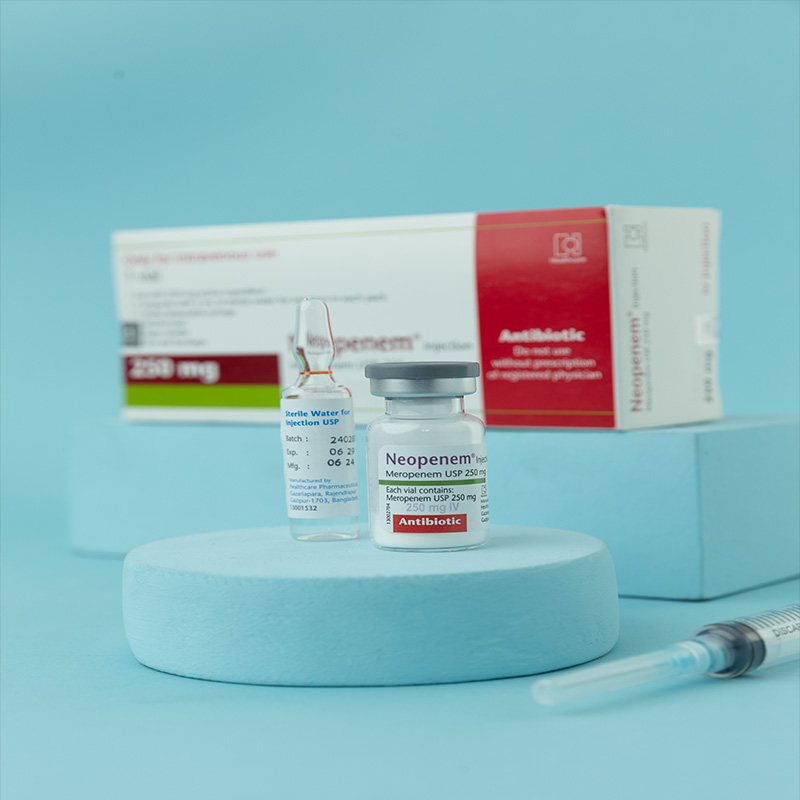
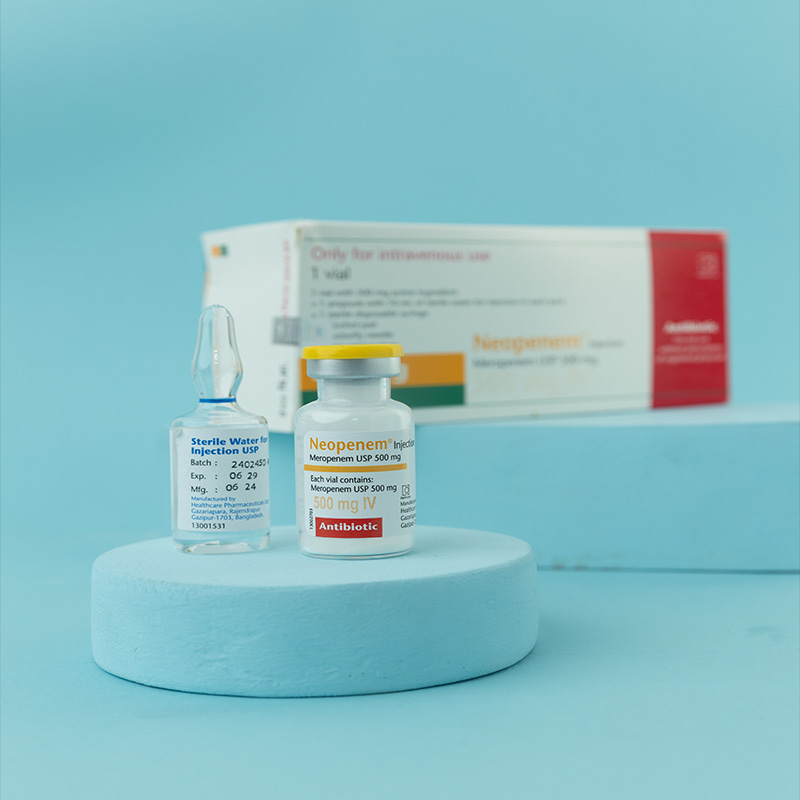
Meropenem is a carbapenem antibiotic for parenteral use, structurally similar to imipenem, and stable to human dehydropeptidase-I (DHP-I). It exerts its bactericidal action by interfering with bacterial cell wall synthesis.

Neopenem® is indicated for the treatment of the following infections in adults and children:
Lower Respiratory Tract Infections
Urinary Tract Infections, including complicated infections
Intra-abdominal Infections
Gynaecological Infections, including postpartum infections
Skin and Skin Structure Infections
Meningitis
Septicaemia
Empiric Treatment: Including initial monotherapy for presumed bacterial infections in neutropenic patients.
Effective for polymicrobial infections due to its broad-spectrum bactericidal activity.

Adults
Usual dose: 500 mg to 1 g IV every 8 hours based on infection type, severity, pathogen susceptibility, and patient condition.
Exceptions:
Febrile Neutropenic Episodes: 1 g IV every 8 hours.
Meningitis: 2 g IV every 8 hours.
Method of administration: IV bolus over ~5 minutes or IV infusion over 15-30 minutes.
Elderly
No dosage adjustment needed if renal function is normal or creatinine clearance >50 mL/min.
Children (Over 3 months)
Dose: 10-40 mg/kg IV every 8 hours based on infection type, severity, and pathogen susceptibility.
Exceptions:
Febrile Neutropenic Episodes: 20 mg/kg every 8 hours.
Meningitis: 40 mg/kg every 8 hours.
Method of administration: Same as for adults.
Adults with Renal Impairment
Dosage adjustments based on creatinine clearance:
Creatinine Clearance (mL/min) Dose Frequency
26-50 1 unit dose Every 12 hours
10-25 ½ unit dose Every 12 hours
<10 ½ unit dose Every 24 hours
Haemodialysis: Administer recommended dose post-dialysis.

No dosage adjustment needed.

Hypersensitivity to meropenem, carbapenems, or beta-lactam antibiotics.
Warnings and Precautions
Hypersensitivity: Patients with a history of allergies to carbapenems or beta-lactam antibiotics may also react to Neopenem®.
Pseudomembranous Colitis: Consider diagnosis in patients with diarrhea during therapy.
Drug Interaction: May reduce serum valproic acid levels, potentially leading to subtherapeutic levels.
Special Populations
Children (<3 months): Safety and efficacy not established.
Liver Disease: Monitor liver function in patients with pre-existing liver disorders.

Pregnancy: Category B. Use only if potential benefits justify risks to the fetus.
Lactation: Detected at low concentrations in animal milk. Avoid use unless benefits outweigh risks to the infant.

Common:
Nausea, vomiting, diarrhea, rash, headache, and inflammation at the injection site.
Rare/Serious:
Pseudomembranous colitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, anaphylaxis, convulsions, agranulocytosis, hemolytic anemia, and angioedema.

Probenecid: Inhibits renal excretion, increasing half-life and plasma levels of meropenem. Co-administration is not recommended.
Valproic Acid: May reduce serum levels, leading to subtherapeutic effects.

Symptoms: Mild adverse effects consistent with profile; typically resolves with dose reduction or cessation.
Management: Symptomatic treatment. Hemodialysis can remove meropenem and its metabolites.

Store below 25°C. Use freshly reconstituted solutions whenever possible.
Stability: 2 hours at room temperature or 12 hours refrigerated (2-8°C). Do not freeze.