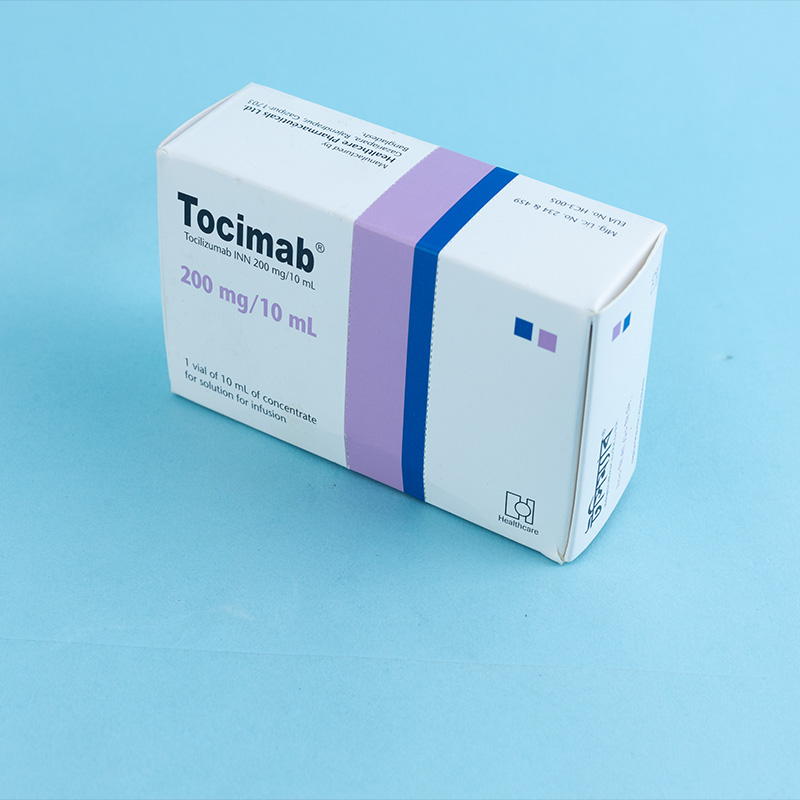
Tocimab is an interleukin-6 (IL-6) receptor antagonist indicated for the treatment of:
Rheumatoid Arthritis (RA): Adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more Disease-Modifying Anti-Rheumatic Drugs (DMARDs).
Polyarticular Juvenile Idiopathic Arthritis (PJIA): Patients 2 years of age and older with active polyarticular juvenile idiopathic arthritis.
Systemic Juvenile Idiopathic Arthritis (SJIA): Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
Cytokine Release Syndrome (CRS): Adults and pediatric patients 2 years of age and older with chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome.
Giant Cell Arteritis (GCA): Adult patients with giant cell arteritis.
Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD): Slowing the rate of decline in pulmonary function in adult patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD).
COVID-19: Emergency use of Tocimab for the treatment of coronavirus disease 2019 in hospitalized adults and pediatric patients (2 years of age and older) who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Tocilizumab is a recombinant humanized anti-human interleukin-6 (IL-6) receptor monoclonal antibody of the immunoglobulin IgG1K (gamma 1, kappa) subclass. Tocilizumab binds to both soluble and membrane-bound IL-6 receptors and has been shown to inhibit IL-6-mediated signaling through these receptors. IL-6 is a pleiotropic pro-inflammatory cytokine produced by a variety of cell types, including T-cells and B-cells, lymphocytes, monocytes, and fibroblasts. IL-6 has been shown to be involved in diverse physiological processes, such as T-cell activation, induction of immunoglobulin secretion, initiation of hepatic acute phase protein synthesis, and stimulation of hematopoietic precursor cell proliferation and differentiation. IL-6 is also produced by synovial and endothelial cells, leading to local production of IL-6 in joints affected by inflammatory processes such as rheumatoid arthritis.
Interruption of dosing may be needed for the management of dose-related laboratory abnormalities, including elevated liver enzymes, neutropenia, and thrombocytopenia.
Rheumatoid Arthritis: Tocilizumab may be used as monotherapy or concomitantly with methotrexate or other non-biologic DMARDs as an intravenous infusion.
Recommended Intravenous Dosage Regimen: The recommended dosage for adult patients given as a 60-minute single intravenous drip infusion is 4 mg per kg of body weight every 4 weeks, followed by an increase to 8 mg per kg every 4 weeks based on clinical response.
Dose reduction from 8 mg per kg to 4 mg per kg is recommended for management of certain dose-related laboratory changes, including elevated liver enzymes, neutropenia, and thrombocytopenia.
Doses exceeding 800 mg per infusion are not recommended for RA patients.
Polyarticular Juvenile Idiopathic Arthritis (PJIA): Tocilizumab may be used as an intravenous infusion or as a subcutaneous injection alone or in combination with methotrexate. Do not change the dose based solely on a single body weight measurement, as weight may fluctuate.
Recommended Intravenous Dosage Regimen:
Patients <30 kg: 10 mg/kg every 4 weeks.
Patients ≥30 kg: 8 mg/kg every 4 weeks.
Systemic Juvenile Idiopathic Arthritis (SJIA): Tocilizumab may be used as an intravenous infusion or as a subcutaneous injection alone or in combination with methotrexate. Do not change the dose based solely on a single body weight measurement, as weight may fluctuate.
Recommended Intravenous Dosage Regimen: Patients <30 kg: 12 mg/kg every 2 weeks , Patients ≥30 kg: 8 mg/kg every 2 weeks.
Coronavirus Disease 2019 (COVID-19):
Recommended Intravenous Dosage Regimen:
Patients <30 kg: 12 mg/kg as a single 60-minute IV infusion.
Patients ≥30 kg: 8 mg/kg as a single 60-minute IV infusion.
If clinical signs or symptoms worsen or do not improve after the first dose, one additional infusion may be administered at least 8 hours after the initial infusion. The maximum dose is 800 mg per infusion.
Cytokine Release Syndrome (CRS): Use only the intravenous route for the treatment of CRS.
Recommended Intravenous CRS Dosage: Patients <30 kg: 12 mg/kg (alone or with corticosteroids).
Patients ≥30 kg: 8 mg/kg (alone or with corticosteroids).
If no clinical improvement occurs after the first dose, up to three additional doses may be administered with at least an 8-hour interval.
Giant Cell Arteritis (GCA):Recommended Dosage: 6 mg/kg IV every 4 weeks in combination with glucocorticoids. Tocilizumab can be used alone after glucocorticoid discontinuation. Doses exceeding 600 mg per infusion are not recommended.
Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD): Recommended Dosage: 162 mg once weekly as a subcutaneous injection. Intravenous administration is not approved for SSc-ILD.
Exercise caution when co-administering Tocimab with CYP3A4 substrate drugs where a decrease in effectiveness is undesirable (e.g., oral contraceptives, lovastatin, atorvastatin). The effect on CYP450 enzyme activity may persist for several weeks after stopping therapy.
Tocilizumab is contraindicated in patients with known hypersensitivity to the drug.
Common adverse reactions (≥5% incidence): Upper respiratory tract infections, nasopharyngitis, headache, hypertension, increased ALT, injection site reactions.
Most common adverse reactions in COVID-19 patients (>3% incidence): Constipation, anxiety, diarrhea, insomnia, hypertension, nausea.
Based on animal data, Tocimab may cause fetal harm.
In COVID-19 cases, use during pregnancy only if the potential benefit justifies the risk.
Breastfeeding individuals with COVID-19 should follow clinical guidelines to prevent infant exposure.

Serious Infections: Do not administer Tocimab during an active infection. Interrupt treatment if a serious infection develops.
Tuberculosis: Evaluate patients for TB risk factors and test for latent infection before initiating Tocimab.
Hepatotoxicity: Monitor for liver injury signs. Modify or discontinue if necessary.
Gastrointestinal Perforation: Use with caution in high-risk patients.
Laboratory Monitoring: Monitor neutrophils, platelets, lipids, and liver function.
Live Vaccines: Avoid use.
Avoid Use with Biological DMARDs.

Pediatric Use: Safety in patients <2 years is not established.
Geriatric Use: Increased infection risk in elderly patients.
Hepatic/Renal Impairment: Use caution; no established safety data.

Monitor patients for adverse reactions and provide symptomatic treatment as needed.
Store at 2°C to 8°C in a dry place. Do not freeze.