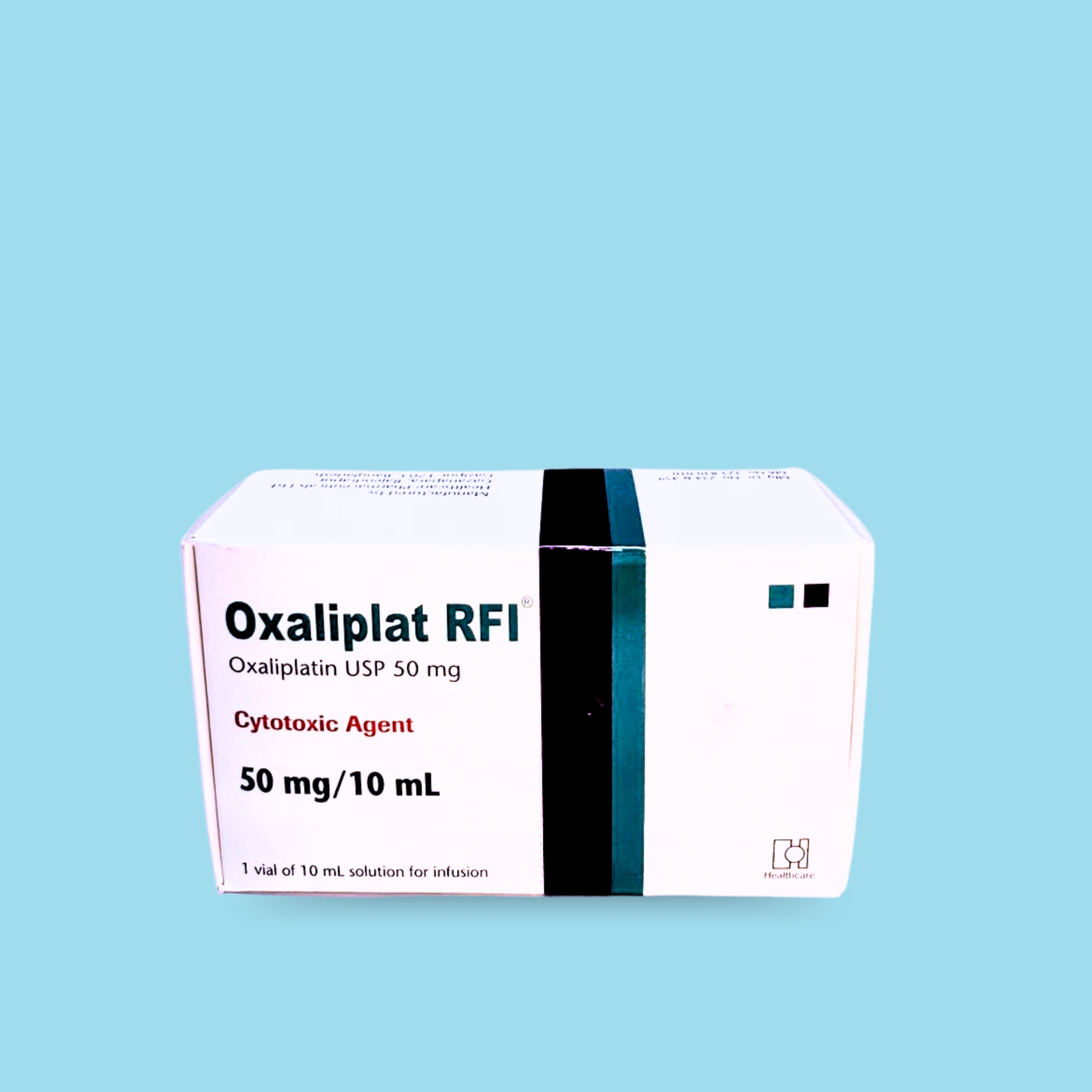
Oxaliplatin is a platinum-based drug used in combination with infusional 5-fluorouracil/leucovorin, which is indicated for:
Adjuvant treatment of stage III colon cancer in patients who have undergone complete resection of the primary tumor.
Treatment of advanced colorectal cancer.
Administer Oxaliplatin in combination with 5-fluorouracil/leucovorin every 2 weeks:
Day 1: Oxaliplatin 85 mg/m² intravenous infusion in 250-500 mL 5% Dextrose Injection, USP, and leucovorin 200 mg/m² intravenous infusion in 5% Dextrose Injection, USP, both given over 120 minutes at the same time in separate bags using a Y-line.
Followed by 5-fluorouracil 400 mg/m² intravenous bolus given over 2-4 minutes.
Followed by 5-fluorouracil 600 mg/m² intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion.
Day 2: Leucovorin 200 mg/m² intravenous infusion over 120 minutes, followed by 5-fluorouracil 400 mg/m² IV bolus given over 2-4 minutes, followed by 5-fluorouracil 600 mg/m² intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion.
Dose Adjustments: Reduce the dose of Oxaliplatin to 75 mg/m² (adjuvant setting) or 65 mg/m² (advanced colorectal cancer) if there are persistent Grade 2 neurosensory events that do not resolve.
Reduce the dose after recovery from:
Grade 3/4 gastrointestinal toxicities (despite prophylactic treatment).
Grade 4 neutropenia.
Grade 3/4 thrombocytopenia.
Delay the next dose until neutrophils ≥1.5 × 10⁹/L and platelets ≥75 × 10⁹/L.
Known allergy to Oxaliplatin or other platinum compounds.
Allergic Reactions: Monitor for rash, urticaria, erythema, pruritus, bronchospasm, and hypotension.
Neuropathy: Reduce the dose or discontinue Oxaliplatin if necessary.
Pulmonary Toxicity: May need to discontinue Oxaliplatin until interstitial lung disease or pulmonary fibrosis is ruled out.
Hepatotoxicity: Monitor liver function tests.
Pregnancy: Can cause fetal harm when administered to a pregnant woman. Women should be advised of the potential risks to the fetus.
The most common adverse reactions (incidence ≥40%) include:
Peripheral sensory neuropathy, neutropenia, thrombocytopenia, anemia, nausea, increased transaminases and alkaline phosphatase, diarrhea, vomiting, fatigue, and stomatitis.
Other adverse reactions, including serious reactions, have been reported.
No specific cytochrome P-450 based drug interaction studies have been conducted.
No pharmacokinetic interaction was observed between 85 mg/m² Oxaliplatin and 5-fluorouracil/leucovorin in patients treated every 2 weeks.
Doses of 130 mg/m² Oxaliplatin every 3 weeks resulted in a 20% increase in 5-fluorouracil plasma concentrations.
Since platinum-containing species are eliminated primarily through the kidneys, clearance may be reduced by nephrotoxic drugs, although this has not been specifically studied.
Pregnancy Category D
Nursing Mothers
It is unknown whether Oxaliplatin or its derivatives are excreted in human milk.
Due to the potential for serious adverse reactions in nursing infants, a decision should be made to either discontinue nursing or discontinue the drug, considering the importance of the drug to the mother.
Pediatric Use
The effectiveness of Oxaliplatin in children has not been established.
Two Phase 1 and two Phase 2 trials in 235 pediatric patients (ages 7 months to 22 years) with solid tumors showed no significant activity.
Geriatric Use
No significant effect of age on the clearance of ultrafilterable platinum has been observed.
Patients with Renal Impairment
Increased plasma levels of unbound platinum have been observed in patients with renal impairment.
Caution and close monitoring are required.
Starting dose adjustments:
Mild impairment (Creatinine Clearance: 50-80 mL/min): No dose reduction needed.
Moderate impairment (Creatinine Clearance: 30-49 mL/min): No dose reduction needed.
Severe impairment (Creatinine Clearance < 30 mL/min): Reduce the starting dose [see Dosage and Administration].
There is no known antidote for Oxaliplatin overdose.
Possible overdose effects include:
Thrombocytopenia, hypersensitivity reactions, myelosuppression, nausea, vomiting, diarrhea, and neurotoxicity.
Several cases of overdose have been reported, with adverse effects including:
Grade 4 thrombocytopenia (<25,000/mm³) without bleeding.
Anemia, sensory neuropathy (paresthesia, dysesthesia, laryngospasm, facial muscle spasms).
Gastrointestinal disorders (nausea, vomiting, stomatitis, flatulence, abdominal distension, Grade 4 intestinal obstruction, Grade 4 dehydration).
Dyspnea, wheezing, chest pain, respiratory failure, severe bradycardia, and death.
Patients suspected of receiving an overdose should be monitored closely, and supportive treatment should be given.