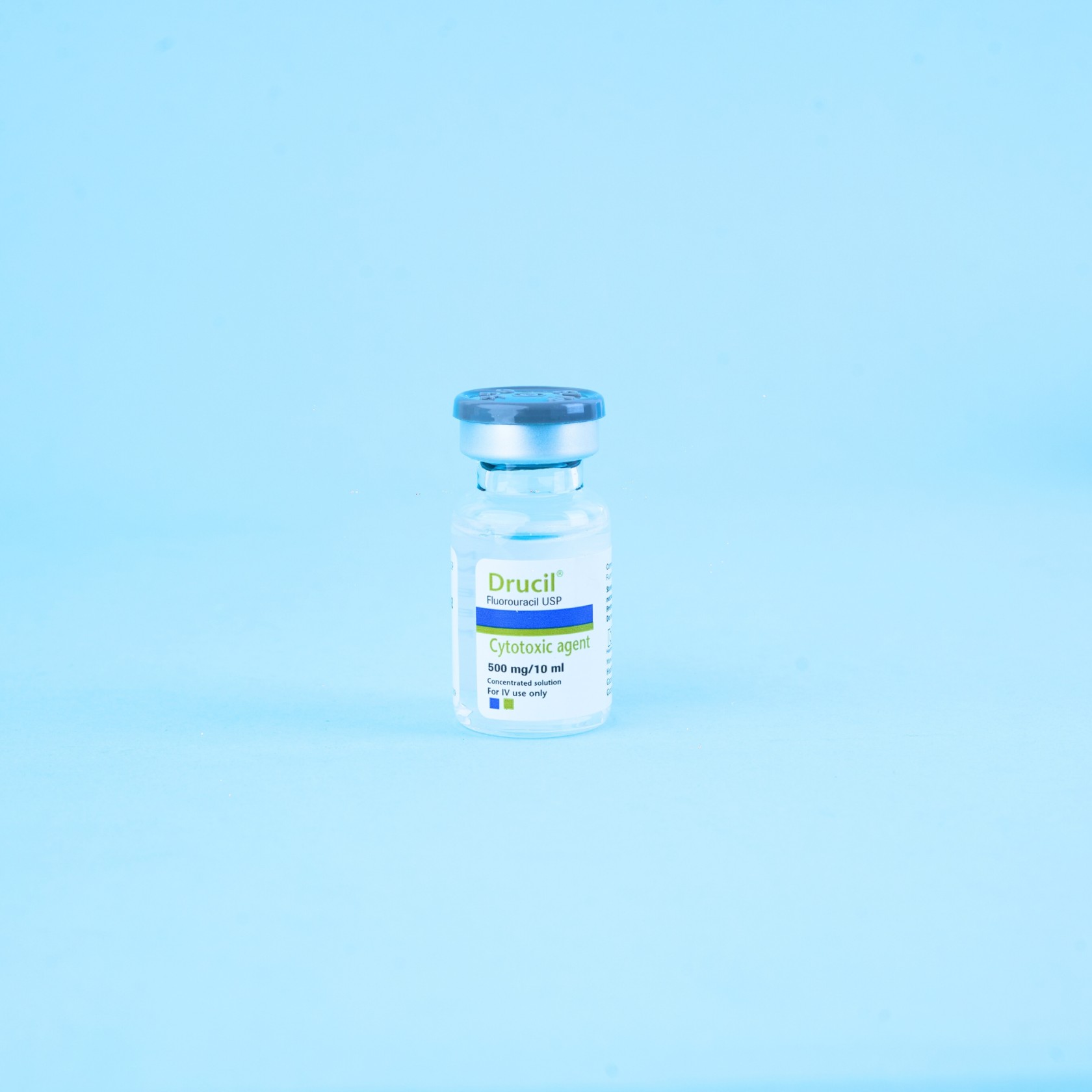
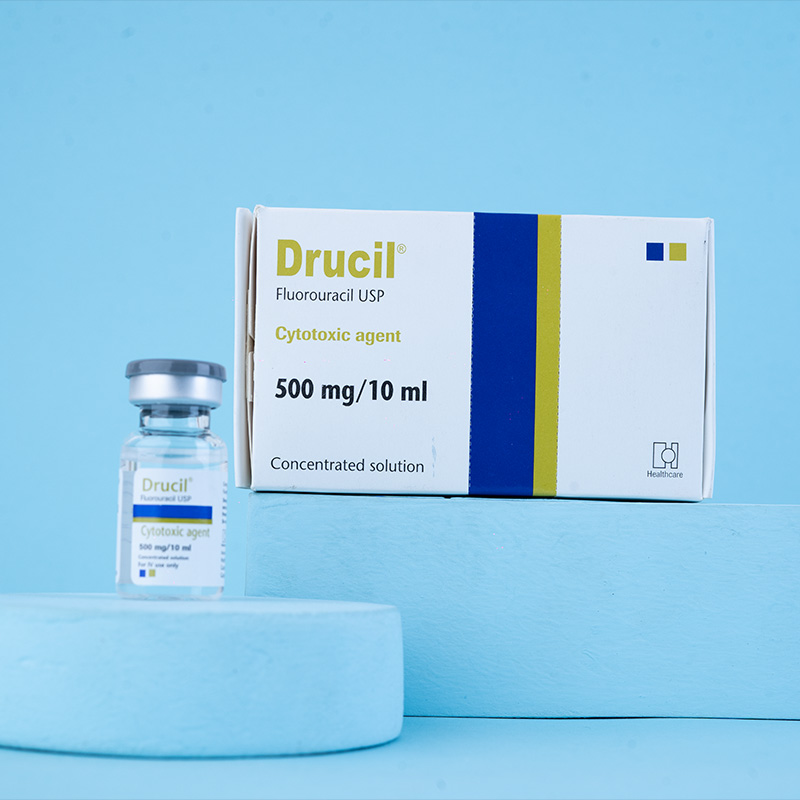
Mechanism of action
Fluorouracil is inactive in mammalian cells but is converted into the active 5-fluorodeoxyuridine monophosphate (FdUMP) through various metabolic pathways. The drug works by inhibiting the enzyme thymidylate synthase, leading to reduced thymidine synthesis and, consequently, DNA production. The active metabolite FdUMP forms a stable complex with the folate cofactor N-5,10-methylene tetrahydrofolate, which inactivates thymidylate synthase. Fluorouracil, as FdUMP, is also incorporated into RNA, leading to RNA fluorination. The drug's effects are primarily limited to proliferating cells. Although cells in the G2 and S phases are most affected, other cell cycle stages may also experience effects.
Pharmacokinetics
5-Fluorouracil is rapidly metabolized in the liver into biologically inactive metabolites, which are eventually converted into carbon dioxide and eliminated via the lungs. Approximately 80% of 5-Fluorouracil is excreted as respiratory carbon dioxide within 12 hours of administration, while 15% is excreted in the urine.
Therapeutic Indications
Fluorouracil is indicated alone or in combination for the treatment of colorectal carcinoma, carcinoma of the stomach and exocrine pancreas, liver carcinoma, breast carcinoma, bladder carcinoma, lung carcinoma, epithelial ovarian carcinoma, and cervical carcinoma.
Fluorouracil can be administered via rapid intravenous bolus injection or slow infusion. The common schedules for rapid IV injection involve 12–13.5 mg/kg (500 mg/m²) daily for 5 days, repeated every 4 weeks. In the case of slow IV infusion, fluorouracil is diluted in 500 mL of 5% dextrose solution and infused over 2–3 hours for 5 consecutive days. For palliative cancer management, the initial dose is 12 mg/kg IV once daily for 4 consecutive days, with a maximum dose of 800 mg/day. If no toxicity is observed, 6 mg/kg may be administered on days 6, 8, 10, and 12, with no therapy on days 5, 7, 9, or 11. Treatment is discontinued at the end of day 12, even in the absence of toxicity. For poor-risk and malnourished patients, the initial dose is 6 mg/kg/day for 3 days, with a maximum dose of 400 mg/day. If no toxicity is observed, 3 mg/kg may be administered on days 5, 7, and 9, with no therapy on days 4, 6, or 8. Treatment is discontinued at the end of day 9, even in the absence of toxicity.
If no severe toxicity occurs, therapy may be continued using one of the following schedules: (1) repeat the initial course every 30 days after the last day of the previous cycle or (2) once toxicity from the first course subsides, administer 10–15 mg/kg per week as a single dose, with a maximum dose of 1 g/week. Dosage should be adjusted based on the patient's response to prior treatment. For cervical cancer in combination with cisplatin, 1 g/m² IV is administered on Day 1, with the cycle repeated every 21 days.
Usual Pediatric Dose for Malignant Disease
Safety and efficacy in children have not been established. However, treatment may follow adult guidelines in specific cases.
Intra-Arterial Infusion
The usual dose is 5–7.5 mg/kg body weight, dissolved in 20–100 mL of 5% dextrose solution. It is administered over 10–20 days using an infusion pump.
Combination with Radiation Therapy
The usual adult dose is 5–10 mg/kg body weight daily, administered either systemically or intra-arterially.
Combination with Other Anticancer Drugs
Fluorouracil is used alone or in combination for adjuvant treatment of breast and gastrointestinal cancers and palliative treatment of inoperable malignancies (e.g., GI tract, breast, liver, genitourinary system, pancreas, head, and neck). The usual dose is 5–10 mg/kg daily, administered either daily or intermittently once to twice weekly via systemic or intra-arterial infusion.
Potentially life-threatening effects include cardiac effects such as rare ischemic cardiac events occurring within hours of the first dose, hematological effects like severe bone marrow suppression within 10 days of treatment (resolving within 3 weeks), and neurological effects, including cerebral ataxia, acute cerebellar syndromes, and myelopathy. Other effects include allergic reactions (e.g., breathing difficulties, facial swelling, or hives), gastrointestinal issues (e.g., severe vomiting, diarrhea, oral ulcers, GI bleeding), hand-foot syndrome (e.g., tingling, pain, redness, swelling, tenderness in hands and feet), conjunctivitis, and tear duct stenosis.
Fluorouracil is contraindicated during pregnancy, especially in the first trimester, due to teratogenic risks. Breastfeeding is not recommended, as excretion in breast milk is unknown.
Fluorouracil has a narrow therapeutic window and requires careful patient monitoring. Treatment should be discontinued immediately if any of the following occur: WBC < 3,500/mm³, platelets < 100,000/mm³, severe stomatitis, diarrhea, GI ulceration, or bleeding.
Cimetidine increases fluorouracil levels by reducing hepatic clearance.
No specific antidote exists; management involves supportive care. Symptoms mimic severe hematological and GI toxicity.
Special Populations
Neonates and children: Safety not established. Elderly patients: Standard dose adjustments based on body weight and height.
Instructions for Use, Handling, and Disposal
5-Fluorouracil is a cytotoxic drug requiring careful handling. Protective equipment (gloves, goggles, masks) must be worn. Skin or mucous membrane exposure should be treated immediately with soap and water. Pregnant personnel should avoid handling. Contaminated materials must be disposed of according to government regulations.
Storage Conditions: Store at ≤ 25°C in the original carton. Do not refrigerate. Protect from light.
Haematological Toxicity Because Gemcitabine is a bone marrow suppressant, anaemia, leukopenia, and thrombocytopenia can occur as a result of administration of Gemcitabine. Myelosuppression is usually mild to moderate and is morepronounced for the granulocyte count. While two-thirds of patients Experience some anaemia, only 7% have haemoglobin levels drop below 8 g/100 mL. While 19% of patients received transfusions, only 0.2% of patients discontinued because of anaemia. The white blood cell count is depressed in 61% of patients, however only 9% of patients experience WBC's below 2000 cells/mm3 and only 0.1% discontinued for leukopenia. Sixty-four percent of patients have reduced granulocyte counts and almost 25% drop below 1000 cells/mm3. Platelet counts are reduced in 21% of patients but only 5% of patients experience counts below 50,000 cells/mm3 and only 0.4% of patients were discontinued due to thrombocytopenia. Previous therapy with cytotoxic agents appears to increase the frequency and severity of the leukopenia, granulocytopenia, and thrombocytopenia. There is no evidence of cumulative haematological toxicity. Anaemia is manageable with the use of conventional transfusions. Dose reduction or omission may be necessary for severe leukopenia or thrombocytopenia (see Dosage and Administration). Rare cases of haemorrhage occurring simultaneously with thrombocytopenia have been reported, but were usually thought to be disease-related. Thrombocythemia is also commonly reported (7.5% of patients), but no patients were discontinued for this event. Febrile neutropenia is also commonly reported.
Hepatic Toxicity: Abnormalities of liver transaminase enzymes occur in about two-thirds of patients, but they are usually mild, non-progressive, and rarely necessitate stopping treatment. Less than 10% of patients experience elevations greater than 5 times normal and only 0.5% of patients were discontinued for abnormalities in liver function. One patient was discontinued for liver failure, but the assessment was complicated by a history of chronic alcoholism. Alanine transaminase (ALT) effects decline over time despite continued treatment. Elevations of alkaline phosphatase greater than 5 times normal occurred in 6.6% of patients but may have been due to bone disorders. Bilirubin values greater than 5 times normal were observed in 1.5% of patients, but ninety percent of patients had normal bilirubin levels.
Gastrointestinal: Nausea, and nausea accompanied by vomiting are each reported in about one-third of patients, respectively. This adverse event requires therapy in about 20% of patients, is rarely dose-limiting, and is easily manageable with standard antiemetics. Only 0.9% of patients report intractable vomiting and only 0.9% of patients discontinued due to nausea and vomiting. Diarrhoea and stomatitis are commonly reported. Diarrhoea (transient to tolerable) was reported by 7% of patients. Intolerable diarrhoea requiring therapy was reported in 0.5% of patients. No patients discontinued treatment because of diarrhoea.
Genito-Urinary Toxicity: Mild proteinuria and haematuria are reported in approximately half the patients, but are rarely clinically significant, and are not usually associated with any change in serum creatinine or blood urea nitrogen. However, a fewcases (0.6% of patients) of renal failure of uncertain aetiology have been reported hence Gemcitabine should be used with caution in patients with impaired renal function (see Precautions). Rare cases (0.4%) of possible haemolytic uraemic syndrome have been reported. Cumulative renal toxicity has not been observed.
Pulmonary Toxicity: Dyspnoea occurring within hours following Gemcitabine injection is reported by approximately 10% of patients. This dyspnoea is usually mild and short-lived, rarely dose-limiting, and usually abates spontaneously without any specific therapy. The mechanism of this toxicity is unknown and the relationship to Gemcitabine is not clear. Only 0.6% of patients discontinued due to dyspnoea and only 0.1% of these were believed to be medicine-related. Interstitial pneumonitis has been reported infrequently.
Allergic Toxicity: A rash is seen in approximately 25% of patients and is associated with pruritus in about 10% of patients. The rash is usually mild, not dose-limiting, and responds to local therapy. Desquamation, vesiculation, and ulceration have been reported rarely. Discontinuations for cutaneous toxicity were reported for only 0.3% of patients. Gemcitabine is well tolerated during the infusion with only a few cases of injection site reaction reported. Gemcitabine does not appear to be a vesicant. There have been no reports of injection site necrosis. Bronchospasm is usually mild and transient, but parenteral therapy may be required. Gemcitabine should not be administered to patients with a known hypersensitivity to the medicine (see Contraindications).
Neurotoxicity: Mild to moderate somnolence occurs in approximately 10% of patients. Only 0.1% of patients discontinued for somnolence. Asthenia is frequently reported with other flu symptoms but is also reported as an isolated symptom. Asthenia was cause for discontinuation by 1.4% of patients. Paresthesias are reported in 3.4% of patients, but only 0.2% report these as severe.
Flu Symptoms: An entity resembling influenza is reported by approximately 20% of patients. This is usually mild, short-lived, and rarely dose-limiting with 1.5% of patients reporting this to be severe. Fever, headache, back pain, chills, myalgia, asthenia, and anorexia are the most commonly reported symptoms. Cough, rhinitis, malaise, sweating and insomnia are also commonly reported. Fever and asthenia are also reported frequently as isolated symptoms. The mechanism of this toxicity is unknown. Reports received indicate that paracetamol may produce symptomatic relief. Only 0.1% of patients reported discontinuation because of the flu symptoms. The percentages of patients who discontinued for fever, malaise, or myalgia are reported as 0.4%, 0.3% and 0.1% respectively.
Oedema/Peripheral Oedema: Oedema/peripheral oedema is reported by approximately 30% of patients. Some cases of facial oedema have also been reported. Pulmonary oedema was reported infrequently (1%). Oedema/peripheral oedema is usually mild to moderate, rarely dose-limiting, is sometimes reported as painful and is usually reversible after stopping Gemcitabine treatment. The mechanism of this toxicity is unknown. However, it was not associated with any evidence of cardiac, renal or hepatic failure. Oedema resulted in the discontinuation of 0.7% of patients.
Alopecia: Overall, 86.7% of patients had no hair loss at all. Minimal to moderate hair loss was reported by 13% of patients. Only 0.5% of patients reported complete but reversible alopecia.
Other Adverse Effects :
The following adverse effects are also reported. Oral toxicity mainly described as soreness or erythema occurred in 7% of patients, however this only required a liquid diet in 0.2% of patients. Mild constipation is reported by 6% of patients. A few cases of hypotension have been reported with only 0.1% of patients discontinued for this event. Irrespective of medicine causality, some cases of myocardial infarction, congestive heart failure, and arrhythmia have been reported in studies. Radiation toxicity has been reported (see Interactions section). Hypersensitivity: anaphylactoid reaction has been reported very rarely.
Cardiovascular: Heart failure has been reported very rarely. Arrhythmias, predominantly supraventricular in nature, have been reported.
Vascular: Clinical signs of peripheral vasculitis and gangrene have been reported very rarely.
Skin and Appendages: Severe skin reactions, including desquamation and bullous skin eruptions, have been reported very rarely.
Injury, Poisoning and Procedural Complications: Radiation toxicity and radiation recall reactions have been reported.
Gemcitabine is contraindicated in those patients with a known hypersensitivity to the medicine or any of the excipients in the medicinal product.
Prolongation of the infusion time beyond 60 minutes and more frequent than weekly dosing have been shown to increase toxicity. Gemcitabine can suppress bone marrow function as manifested by leukopenia, thrombocytopenia and anemia, and myelosuppression is usually the dose-limiting toxicity. Patients should be monitored for myelosuppression during therapy. See Dosage and Administration for recommended dose adjustments. Hemolytic-Uremic Syndrome (HUS) has been reported rarely with the use of Gemcitabine
Pregnancy: Pregnancy Category D. Gemcitabine can cause fetal harm when administered to a pregnant woman. Gemcitabine is embryotoxic causing fetal malformations (cleft palate, incomplete ossification) at doses of 1.5mg/kg/day in mice (about 1/200 the recommended human dose on a mg/m2 basis). Gemcitabine is fetotoxic causing fetal malformations (fused pulmonary artery, absence of gall bladder) at doses of 0.1 mg/kg/day in rabbits (about 1/600 the recommended human dose on a mg/m2 basis). Embryotoxicity was characterized by decreased fetal viability, reduced live litter sizes, and developmental delays. There are no studies of Gemcitabine in pregnant women. If Gembine® is used during pregnancy, or if the patient becomes pregnant while taking Gembine® , the patient should be apprised of the potential hazard to the fetus.
General: Patients receiving therapy with Gemcitabine should be monitored closely by a physician experienced in the use of cancer chemotherapeutic agents. Most adverse events are reversible and do not need to result in discontinuation, although doses may need to be withheld or reduced. There was a greater tendency in women, especially older women, not to proceed to the next cycle.
Laboratory Tests: Patients receiving Gemcitabine should be monitored prior to each dose with a complete blood count (CBC), including differential and platelet count. Suspension or modification of therapy should be considered when marrow suppression is detected Laboratory evaluation of renal and hepatic function should be performed prior to initiation of therapy and periodically thereafter. Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term animal studies to evaluate the carcinogenic potential of Gemcitabine have not been conducted. Gemcitabine induced forward mutations in vitro in a mouse lymphoma (L5178Y) assay and was clastogenic in an in vivo mouse micronucleus assay. Gemcitabine was negative when tested using the Ames, in vivo sister chromatid exchange, and in vitro chromosomal aberration assays, and did not cause unscheduled DNA synthesis in vitro. Gemcitabine I.P. doses of 0.5 mg/kg/day (about 1/700 the human dose on a mg/m2 basis) in male mice had an effect on fertility with moderate to severe hypospermatogenesis, decreased fertility, and decreased implantations. In female mice fertility was not affected but maternal toxicities were observed at 1.5 mg/kg/day I.V. (about 1/200 the human dose on a mg/m2 basis) and fetotoxicity or embryolethality was observed at 0.25 mg/kg/day I.V. (about 1/1300 the human dose on a mg/m2 basis).
Nursing Mothers: It is not known whether Gemcitabine or its metabolites are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from Gemcitabine in nursing infants, the mother should be warned and a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother and the potential risk to the infant.
Elderly Patients: Gemcitabine clearance is affected by age. There is no evidence, however, that unusual dose adjustments, (i.e., other than those already recommended in the Dosage and Administration section) are necessary in patients over 65, and, in general adverse reaction rates in the single-agent safety database of 979 patients were similar in patients above and below 65. Grade 3 / 4 thrombocytopenia was more common in the elderly.
Gender: Gemcitabine clearance is affected by gender. In the single agent safety database (N=979 patients), however, there is no evidence that unusual dose adjustments (i.e., other than those already recommended in the Dosage and Administration section) are necessary in women. In general, in single agent studies of Gemcitabine adverse reaction rates were similar in men and women, but women, especially older women, were more likely not to proceed to a subsequent cycle and to experience grade 3 / 4 neutropenia and thrombocytopenia.
Pediatric Patients: Gemcitabine has not been studied in pediatric patients. Safety and effectiveness in pediatric patients have not been established. Patients with Renal or Hepatic Impairment: Gemcitabine should be used with caution in patients with preexisting renal impairment or hepatic insufficiency. Gemcitabine has not been studied in patients with significant renal or hepatic impairment.
Drug Interactions: No confirmed interactions have been reported with the use of Gemcitabine. No specific drug interaction studies have been conducted. Radiation Therapy: Safe and effective regimens for the administration of Gemcitabine with therapeutic doses of radiation have not yet been determined.
Concurrent (given together or 7 days apart): Based on the result of preclinical studies and clinical trials, Gemcitabine has radiosensitising activity. In a single trial, where Gemcitabine at a dose of 1,000mg/m2 was administered concurrently for up to 6 consecutive weeks with therapeutic thoracic radiation to patients with non-small cell lung cancer, significant toxicity in the form of severe and potentially life-threatening mucositis, especially oesophagitis and pneumonitis, was observed, particularly in patients receiving large volumes of radiotherapy (median treatment volumes 4,795 cm3). Studies done subsequently have suggested that it is feasible to administer Gemcitabine at lower doses with concurrent radiotherapy with predictable toxicity, such as a Phase II study in non-small cell lung cancer. Thoracic radiation doses of 66Gy were administered with Gemcitabine (600mg/m2, four times) and cisplatin (80mg/m2, twice) during 6 weeks. The optimum regimen for safe administration of Gemcitabine with therapeutic doses of radiation has not yet been determined.
Store at temperature not exceeding 25 0 C in a dry place. Protect from light and moisture. Do not freeze