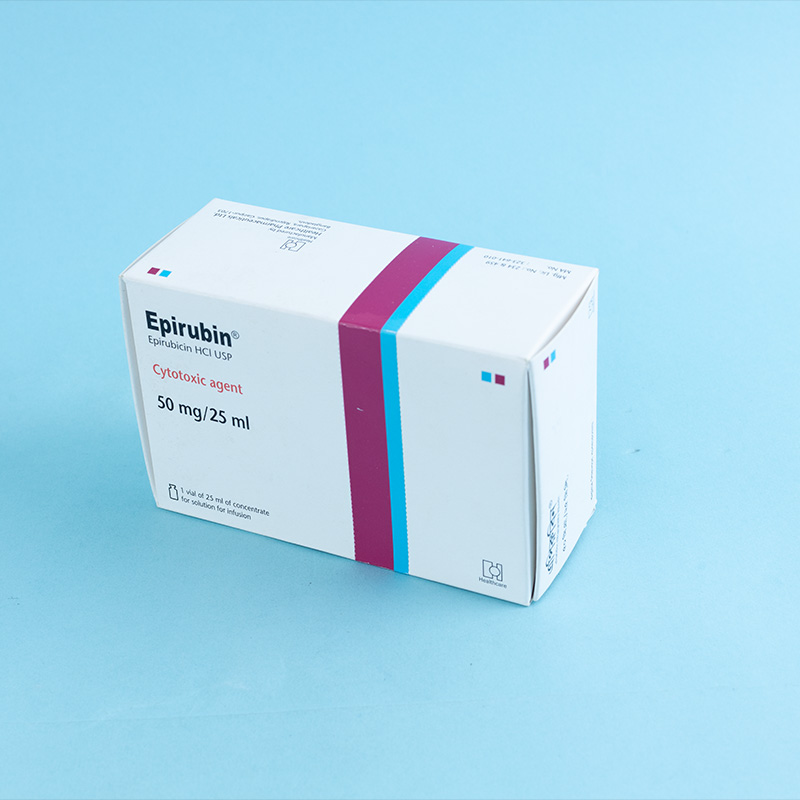
Epirubicin is an anthracycline cytotoxic agent used in chemotherapy. While the exact mechanism of action is not fully understood, it primarily acts by intercalating into DNA, inhibiting nucleic acid (DNA and RNA) and protein synthesis, thereby preventing cancer cell replication and inducing cell death.
Distribution
After intravenous administration, Epirubicin is rapidly distributed into tissues. Approximately 77% of the drug binds to plasma proteins, mainly albumin, with binding unaffected by drug concentration.
Metabolism
Epirubicin undergoes extensive and rapid metabolism in the liver and other organs, including red blood cells. Major metabolic pathways include:
Reduction of the C-13 keto group to form epirubicinol.
Glucuronic acid conjugation of epirubicin and epirubicinol.
Hydrolytic loss of the amino sugar, forming doxorubicin and doxorubicinol aglycones.
Redox loss of the amino sugar, forming 7-deoxy-doxorubicin and 7-deoxy-doxorubicinol aglycones.
Epirubicinol retains about one-tenth of Epirubicin’s cytotoxic activity but remains at lower plasma levels, reducing its in vivo cytotoxic potential.
Excretion
Epirubicin and its metabolites are primarily excreted through bile (60%), with a smaller proportion eliminated via urine (27%). Excretion rates may be altered in patients with hepatic dysfunction.
Age: Elderly women (≥70 years) have a 35% lower plasma clearance than younger females, necessitating close toxicity monitoring.
Gender: No significant clearance differences in males and females ≤50 years.
Race: The impact of race on Epirubicin’s pharmacokinetics has not been studied.
Hepatic Impairment: Clearance is reduced by ~30% in mild impairment and ~50% in moderate impairment. Severe hepatic impairment has not been evaluated.
Renal Impairment: No major pharmacokinetic alterations in patients with creatinine <5 mg/dL, but clearance is reduced by 50% in those with creatinine ≥5 mg/dL. Patients on dialysis have not been studied.
Epirubicin is used as part of adjuvant therapy in patients with axillary node-positive primary breast cancer.
Standard starting dose: 100 to 120 mg/m²
Dosage adjustments are required for patients with hepatic impairment and those with severe renal impairment.

Epirubicin should not be used in patients with:
Baseline neutrophil count <1,500 cells/mm³
Severe myocardial insufficiency, recent myocardial infarction, or severe arrhythmias
Prior cumulative anthracycline exposure exceeding the maximum recommended dose
Hypersensitivity to Epirubicin or related compounds
Severe hepatic dysfunction
Cardiotoxicity: Can cause early (acute) or late (delayed) cardiac complications.
Hepatic Function: Monitor serum bilirubin and AST before and during treatment.
Renal Function: Assess serum creatinine before and during therapy.
Common: Neutropenia, anemia, fatigue, nausea
Rare: Left ventricular ejection fraction (LVEF) reduction, congestive heart failure (CHF)
Pregnancy: Category D – May cause fetal harm.
Lactation: Discontinue breastfeeding before treatment.
Pediatric: Safety and efficacy not established.
Geriatric: Careful toxicity monitoring is recommended in women ≥70 years.