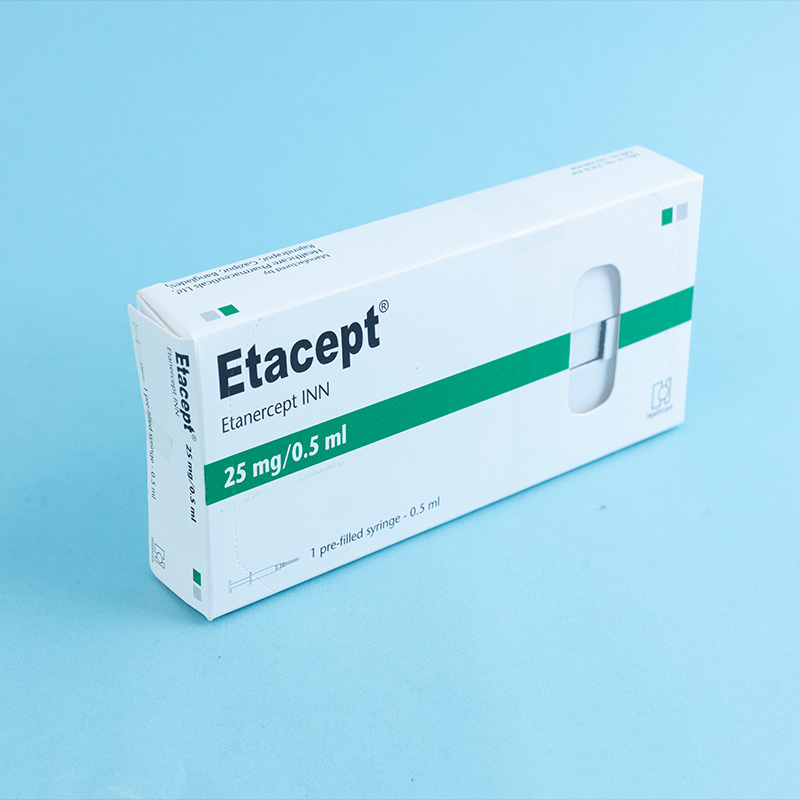
Etanercept is a tumor necrosis factor (TNF) blocker indicated for:
Adults with Rheumatoid Arthritis (RA): Etanercept is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis (RA). Etanercept can be initiated in combination with methotrexate (MTX) or used alone for the treatment of active rheumatoid arthritis (RA) in adults when one or more disease-modifying antirheumatic drugs (DMARDs), including methotrexate (unless contraindicated), have proven inadequate.
Pediatric patients with Juvenile Idiopathic Arthritis (JIA): Etanercept is indicated for the treatment of polyarticular-course juvenile idiopathic arthritis (JIA) in children and adolescents from the age of 2 years when the response to one or more DMARDs has proven inadequate.
Adults with Psoriatic Arthritis (PsA): Etanercept is indicated for reducing signs and symptoms, inhibiting the progression of structural damage of active arthritis, and improving physical function in patients with psoriatic arthritis (PsA). Etanercept can be used in combination with methotrexate (MTX) in patients who do not respond adequately to MTX alone.
Adults with Ankylosing Spondylitis (AS): Etanercept is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis (AS)
Adults with Plaque Psoriasis (PsO): Etanercept is indicated for the treatment of adult patients (18 years or older) with chronic moderate to severe plaque psoriasis (PsO) who are candidates for systemic therapy or phototherapy.
Pediatric patients with Plaque Psoriasis (PsO): Etanercept is indicated for the treatment of chronic severe plaque psoriasis in children and adolescents from the age of 6 years who are inadequately controlled by or are intolerant to systemic therapies or phototherapies.
Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis: Patients aged 18 years or older should take 50 mg of Etanercept per week, administered either once weekly (as one subcutaneous injection using a 50 mg syringe or as two 25 mg injections given at the same time) or 25 mg Etanercept twice weekly (72 to 96 hours apart) as a subcutaneous injection. Methotrexate, glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics may be continued during treatment with Etanercept in adults. A 25 mg once-weekly dosage provides a slower response and may be less effective.
Plaque Psoriasis: The recommended dose of Etanercept is 50 mg once weekly (as one subcutaneous injection using a 50 mg syringe or as two 25 mg injections given at approximately the same time) or 25 mg twice weekly (72 to 96 hours apart) as a subcutaneous injection. Higher responses may be achieved with an initial treatment dose of 50 mg twice weekly for up to 12 weeks, followed, if necessary, by a dose of 50 mg once weekly or 25 mg twice weekly.
Juvenile Idiopathic Arthritis: Children (≥2 to <18 years) should receive 0.4 mg/kg (up to a maximum of 25 mg per dose) twice weekly (72 to 96 hours apart). Glucocorticoids, NSAIDs, or analgesics may be continued during treatment with Etanercept in children. Etanercept has not been studied in children under 2 years of age.
Pediatric Plaque Psoriasis: Children (≥6 to <18 years) should receive 0.8 mg/kg (up to a maximum of 50 mg per dose) once weekly for up to 24 weeks. Treatment should be discontinued in patients who show no response after 12 weeks. If retreatment with Etanercept is necessary, the above guidance on treatment duration should be followed.
Administer Etanercept as subcutaneous injections in the thigh, abdomen, or upper arm. Each new injection should be given at least 3 cm from a previous site, avoiding areas where the skin is tender, bruised, red, or hard. The injection should be performed under the supervision of a qualified healthcare professional.
Before injection, a single-use prefilled syringe should be allowed to reach room temperature (approximately 15–30 minutes). The needle cover should not be removed while allowing the prefilled syringe to reach room temperature. Before disposal of blank prefilled syringes, activate the needle guard to avoid any chance of needle-stick injury.
Hypersensitivity to Etanercept or any component of the formulation
Serious active infections, including chronic or localized infections
Allergic Reactions: If you or your child experiences allergic reactions such as chest tightness, wheezing, dizziness, or rash, stop injecting Etanercept and contact your doctor immediately.
Infections/Surgery: Inform your doctor if you or your child develops a new infection or is about to have major surgery, as treatment may need monitoring.
Tuberculosis Screening: Since cases of tuberculosis have been reported in patients treated with Etanercept, doctors should check for tuberculosis symptoms before starting treatment, including a medical history, chest X-ray, and tuberculin test.
Hepatitis B and C: Patients with a history of hepatitis should inform their doctor, as Etanercept may lead to reactivation of hepatitis B.
Blood Disorders and Nervous System Issues: Seek immediate medical attention if symptoms like persistent fever, sore throat, bruising, or weakness appear.
Like all medicines, Etanercept may cause side effects, though not everyone experiences them.
Very Common (≥1 in 10 patients): Infections (colds, sinusitis, bronchitis, urinary tract infections, skin infections), injection site reactions (bleeding, bruising, redness, itching, pain, swelling).
Common (≥1 in 100 patients): Allergic reactions, fever, itching, and autoantibody formation.
Uncommon (≥1 in 1,000 patients): Serious infections, low platelet count, skin cancer (excluding melanoma), and eye inflammation.
Rare (≥1 in 10,000 patients): Severe allergic reactions, nervous system disorders, tuberculosis, lupus-like syndrome, and worsening heart failure.
The safety of Etanercept during pregnancy and lactation has not been established. Use during pregnancy only if necessary. It is unknown whether Etanercept is excreted in human milk.
The maximum tolerated dose in humans is not established. No dose-limiting toxicities have been observed in clinical trials. There is no known antidote for Etanercept overdose.
● Etanercept should be stored in refrigetor at 2-8 oC.
● Do not freeze.
● Do not shake.
● Keep away from light.
Medicine: Keep out of reach of children