Gemcitabine Hydrochloride is a nucleoside analogue that exhibits antitumor activity. Gemcitabine Hydrochloride is 2'-deoxy-2',2'-difluorocytidine monohydrochloride (β-isomer). The empirical formula for Gemcitabine Hydrochloride is C9H11F2N3O4 HCl. It has a molecular weight of 299.66. Gemcitabine Hydrochloride is a white to off-white solid. It is soluble in water, slightly soluble in methanol, and practically insoluble in ethanol and polar organic solvents.
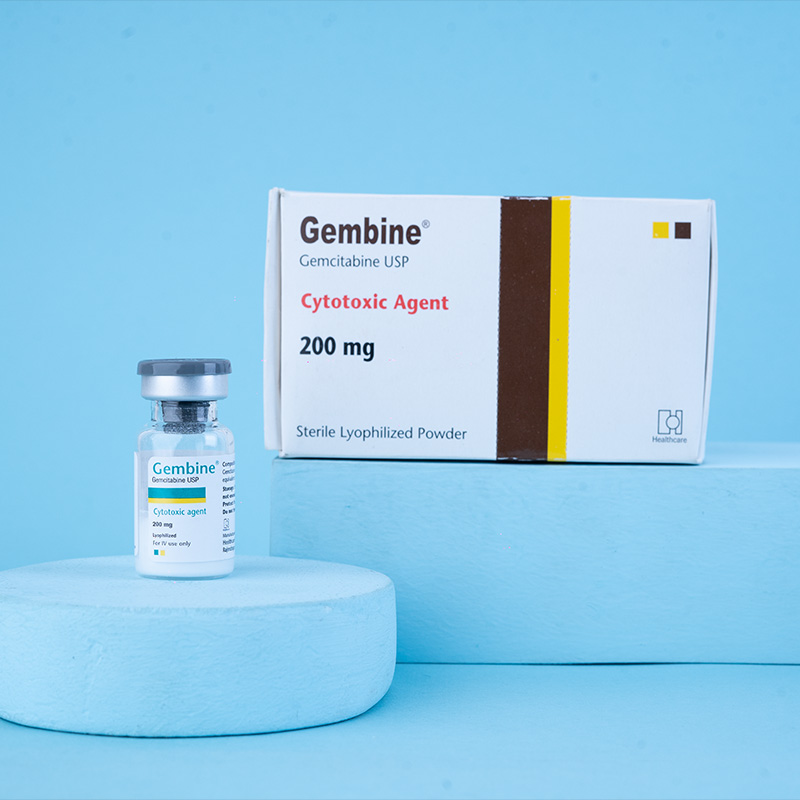
Gembine® Injection 1 g: Each vial contains Gemcitabine Hydrochloride USP 1.1387 g, equivalent to Gemcitabine 1 g (as lyophilized powder).Gembine® Injection 200 mg: Each vial contains Gemcitabine Hydrochloride USP 227.74 mg, equivalent to Gemcitabine 200 mg (as lyophilized powder).
Mechanism of Action: Gemcitabine (dFdC) is metabolized intracellularly by nucleoside kinases to the active diphosphate (dFdCDP) and triphosphate (dFdCTP) nucleosides. The cytotoxic action of Gemcitabine appears to be due to the inhibition of DNA synthesis by two actions of dFdCDP and dFdCTP. First, dFdCDP inhibits ribonucleotide reductase, which is uniquely responsible for catalyzing the reactions that generate the deoxynucleoside triphosphates for DNA synthesis. Inhibition of this enzyme by dFdCDP causes a reduction in the concentrations of deoxynucleosides, especially dCTP. Secondly, dFdCTP competes with dCTP for incorporation into DNA. Likewise, a small amount of Gemcitabine may also be incorporated into RNA. Thus, the reduction in the intracellular concentration of dCTP potentiates the incorporation of dFdCTP into DNA (self-potentiation). DNA polymerase epsilon is essentially unable to remove Gemcitabine and repair the growing DNA strands. After Gemcitabine is incorporated into DNA, one additional nucleotide is added to the growing DNA strands. After this addition, there is essentially a complete inhibition in further DNA synthesis (masked chain termination). After incorporation into DNA, Gemcitabine then appears to induce the programmed cellular death process known as apoptosis.
Pharmacokinetic Properties: Peak plasma concentrations (obtained within 5 minutes of the end of the infusion): 3.2 to 45.5 μg/ml. Volume of distribution of the central compartment: 12.4 l/m2 for women and 17.5 l/m2 for men (inter-individual variability was 91.9%). Volume of distribution of the peripheral compartment: 47.4 l/m2. The volume of the peripheral compartment was not sensitive to gender. Plasma protein binding: Negligible. Systemic clearance: Ranged from 29.2 l/hr/m2 to 92.2 l/hr/m2 depending on gender and age (inter-individual variability was 52.2%). Clearance for women is approximately 25% lower than the values for men. Although rapid, clearance for both men and women appears to decrease with age. For the recommended Gemcitabine dose of 1,000 mg/m2 given as a 30-minute infusion, lower clearance values for women and men should not necessitate a decrease in the Gemcitabine dose. Urinary excretion: Less than 10% is excreted as unchanged drug. Renal clearance: 2 to 7 l/hr/m2. Half-life: Ranged from 42 to 94 minutes depending on age and gender. For the recommended dosing schedule, Gemcitabine elimination should be virtually complete within 5 to 11 hours of the start of the infusion. Gemcitabine does not accumulate when administered once weekly.
Metabolism: Gemcitabine is rapidly metabolized by cytidine deaminase in the liver, kidney, blood, and other tissues. Intracellular metabolism of Gemcitabine produces the Gemcitabine mono-, di-, and triphosphates (dFdCMP, dFdCDP, and dFdCTP), of which dFdCDP and dFdCTP are considered active. These intracellular metabolites have not been detected in plasma or urine. The primary metabolite, 2'-deoxy-2',2'-difluorouridine (dFdU), is not active and is found in plasma and urine.
Therapeutic Indications: Non-Small Cell Lung Cancer: Gemcitabine, in combination with cisplatin, is indicated as a first-line treatment for patients with locally advanced (inoperable Stage IIIA or IIIB) or metastatic (Stage IV) non-small cell lung cancer. Gemcitabine is indicated for the palliative treatment of adult patients with locally advanced or metastatic non-small cell lung cancer.
Pancreatic Cancer: Gemcitabine is indicated for the treatment of adult patients with locally advanced or metastatic adenocarcinoma of the pancreas. Gemcitabine is indicated for patients with 5-FU refractory pancreatic cancer.
Bladder Cancer: Gemcitabine is indicated for the treatment of advanced bladder cancer (muscle-invasive Stage IV tumors with or without metastases) in combination with cisplatin therapy.
Breast Cancer: Gemcitabine, in combination with paclitaxel, is indicated for the treatment of patients with metastatic breast cancer who have relapsed following adjuvant/neoadjuvant chemotherapy. Prior chemotherapy should have included an anthracycline unless clinically contraindicated.
Ovarian Cancer: Gemcitabine, in combination with carboplatin, is indicated for the treatment of patients with recurrent epithelial ovarian carcinoma who have relapsed following platinum-based therapy.
Gemcitabine is for intravenous use only.
Non-Small Cell Lung Cancer (Single-agent Use): Adults — The recommended dose of Gemcitabine is 1000 mg/m2, given by 30-minute intravenous infusion. This should be repeated once weekly for three weeks, followed by a one-week rest period. This four-week cycle is then repeated. Dosage reduction with each cycle or within a cycle may be applied based on the amount of toxicity experienced by the patient.
Non-Small Cell Lung Cancer (Combination Use): Adults — Gemcitabine, in combination with cisplatin, has been investigated using two dosing regimens: a three-week schedule and a four-week schedule.
The three-week schedule used Gemcitabine 1250 mg/m2, given by 30-minute intravenous infusion on days 1 and 8 of each 21-day cycle. Dosage reduction with each cycle or within a cycle may be applied based on the amount of toxicity experienced by the patient.
The four-week schedule used Gemcitabine 1000 mg/m2, given by 30-minute intravenous infusion on days 1, 8, and 15 of each 28-day cycle. Dosage reduction with each cycle or within a cycle may be applied based on the amount of toxicity experienced by the patient.