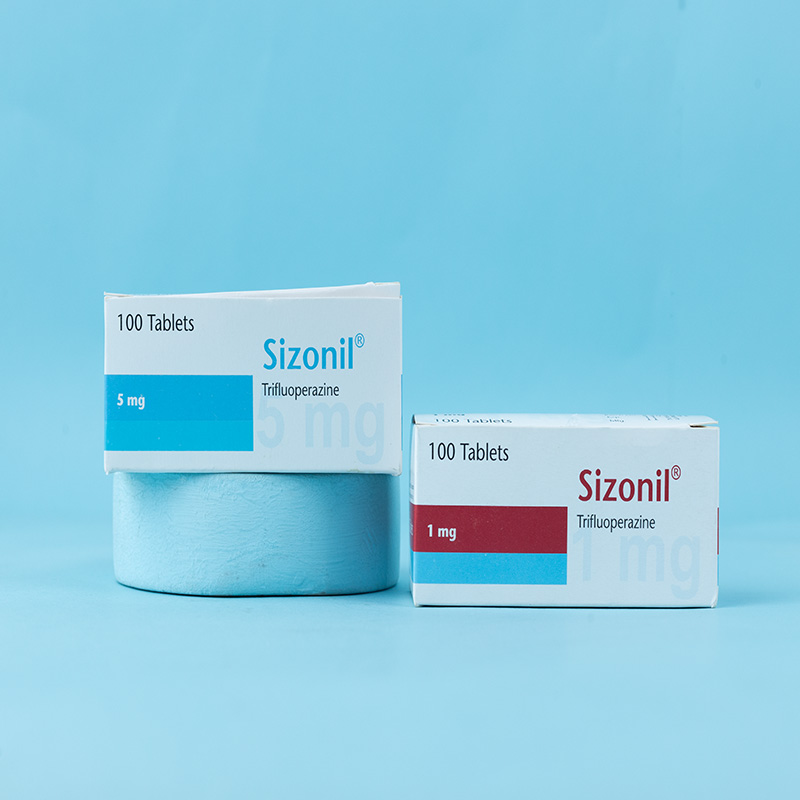
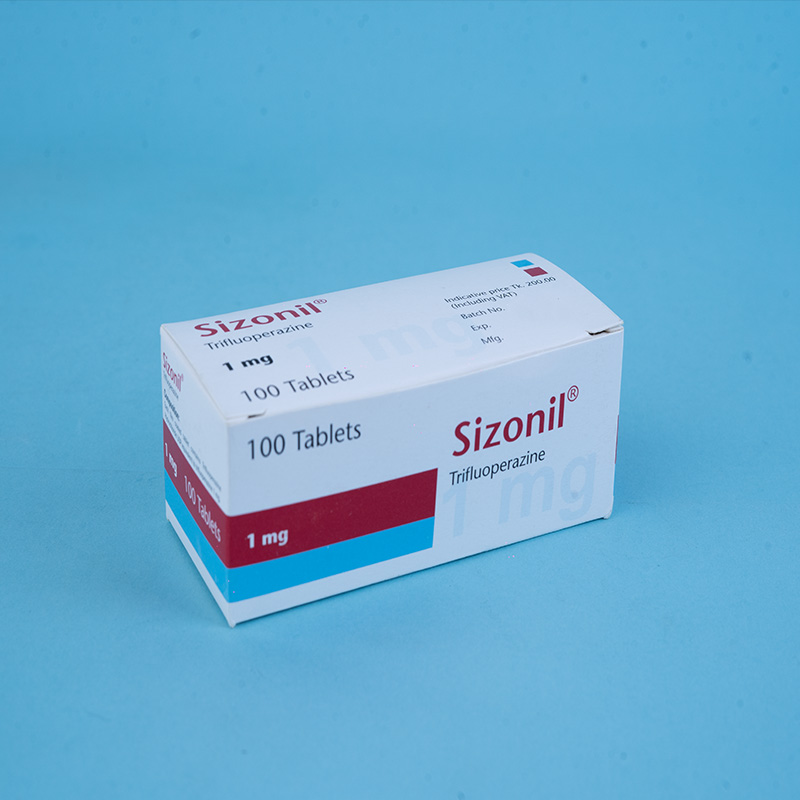
Sizonil® 1 mg: Each film-coated tablet contains Trifluoperazine Hydrochloride USP equivalent to 1 mg of Trifluoperazine.
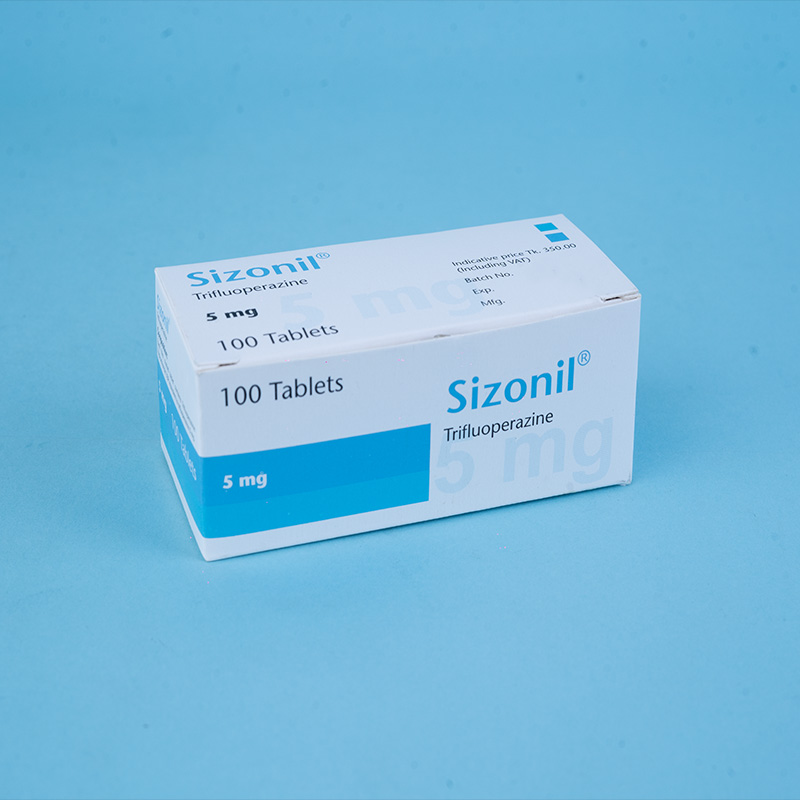
Sizonil® 5 mg: Each film-coated tablet contains Trifluoperazine Hydrochloride USP equivalent to 5 mg of Trifluoperazine.
Trifluoperazine is a phenothiazine compound with multiple pharmacodynamic effects. Its most notable action is antagonism at dopamine receptors in the CNS. This activity in the limbic system and cerebral cortex underpins its antipsychotic effects. It also acts on the medullary chemoreceptor trigger zone, contributing to its antiemetic properties.
Low Dosage
Management of anxiety states, depressive symptoms secondary to anxiety, and agitation (short-term).
Symptomatic treatment of nausea and vomiting.
High Dosage
Treatment and relapse prevention in schizophrenia and other psychoses, particularly paranoid types.
Short-term management of severe psychomotor agitation and dangerously impulsive behavior, including in mentally subnormal individuals.
Comatose patients.
Patients with blood dyscrasias or liver damage.
Hypersensitivity to Trifluoperazine or related compounds.
Elderly patients: Start with a reduced dose due to sensitivity to extrapyramidal and hypotensive effects.
Patients with cardiovascular disease (including arrhythmias) should be treated cautiously.
Monitor patients with angina pectoris as Trifluoperazine may increase activity.
Although no conclusive evidence indicates teratogenic effects in humans, avoid use during pregnancy unless absolutely necessary, especially in the first trimester.
Trifluoperazine is excreted in the milk of lactating dogs; caution is advised in breastfeeding women.
Common: Lassitude, drowsiness, dizziness, dry mouth, blurred vision, transient restlessness, muscular weakness, anorexia, mild postural hypotension, skin reactions, weight gain, and confusion.
Rare: Tachycardia, constipation, urinary hesitancy/retention, hyperpyrexia, and photosensitivity reactions.
Side effects are typically dose-related and transient.
Adults
Low Dosage: 2–4 mg/day in divided doses, up to a maximum of 6 mg/day.
High Dosage: Start at 5 mg twice daily. Gradually increase to 15 mg/day. Further increases (5 mg) may be made at intervals of at least 3 days. Gradually reduce dosage to establish maintenance levels.
Elderly
Start with at least half the usual adult dose.
Children
Low Dosage:
Ages 3–5 years: Up to 1 mg/day in divided doses.
Ages 6–12 years: Maximum of 4 mg/day in divided doses.
High Dosage: Start with ≤5 mg/day in divided doses. Increase cautiously, considering age, weight, and symptom severity.
Symptoms include extrapyramidal effects and hypotension. Treatment involves:
Gastric lavage and supportive care.
Anticholinergic antiparkinsonism drugs for extrapyramidal symptoms.
Fluid replacement for hypotension; noradrenaline if severe or persistent. Avoid adrenaline.
Potentiates CNS depressants such as alcohol, hypnotics, and strong analgesics.
Phenothiazines may antagonize the effects of Guanethidine.
Avoid using Desferrioxamine with Trifluoperazine due to potential prolonged unconsciousness.
Sizonil® 1 mg: Box containing 10 × 10 tablets in Alu-Alu blister packs.
Sizonil® 5 mg: Box containing 10 × 10 tablets in Alu-Alu blister packs.
Store below 30°C in a cool, dry place.
Protect from light.
Keep out of reach of children.