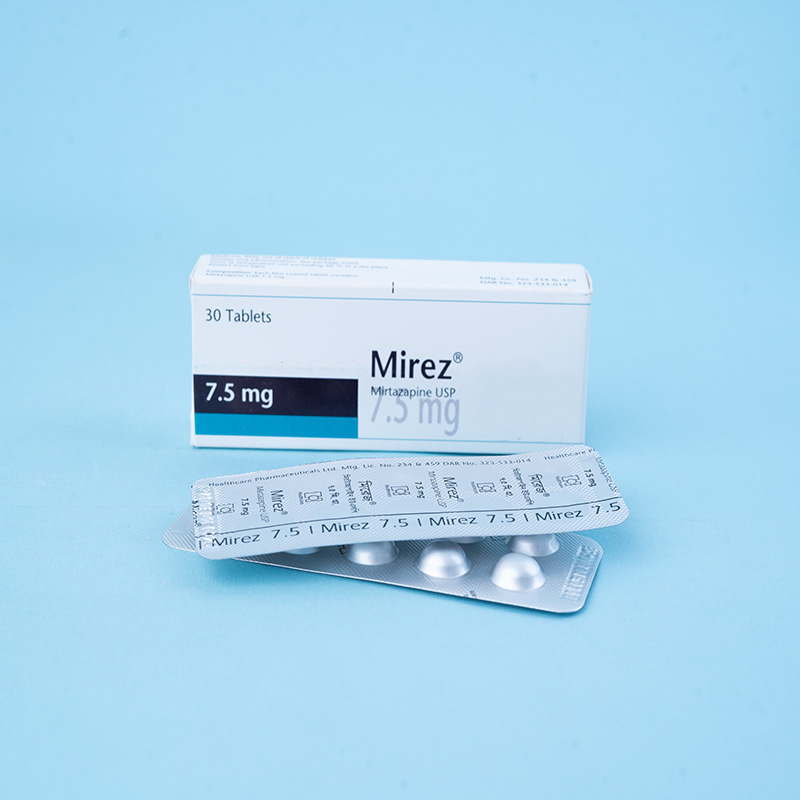
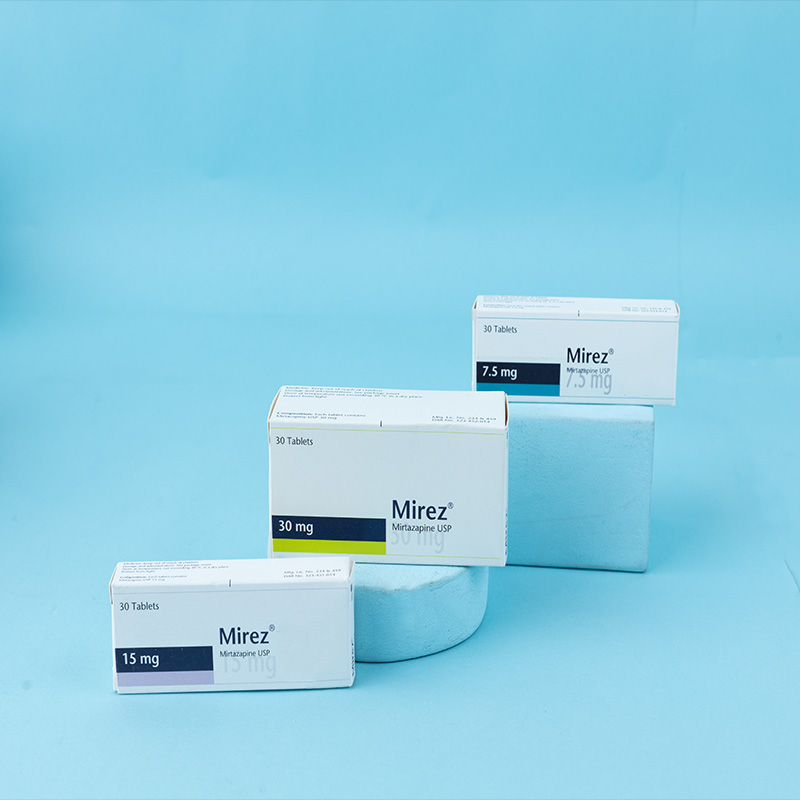
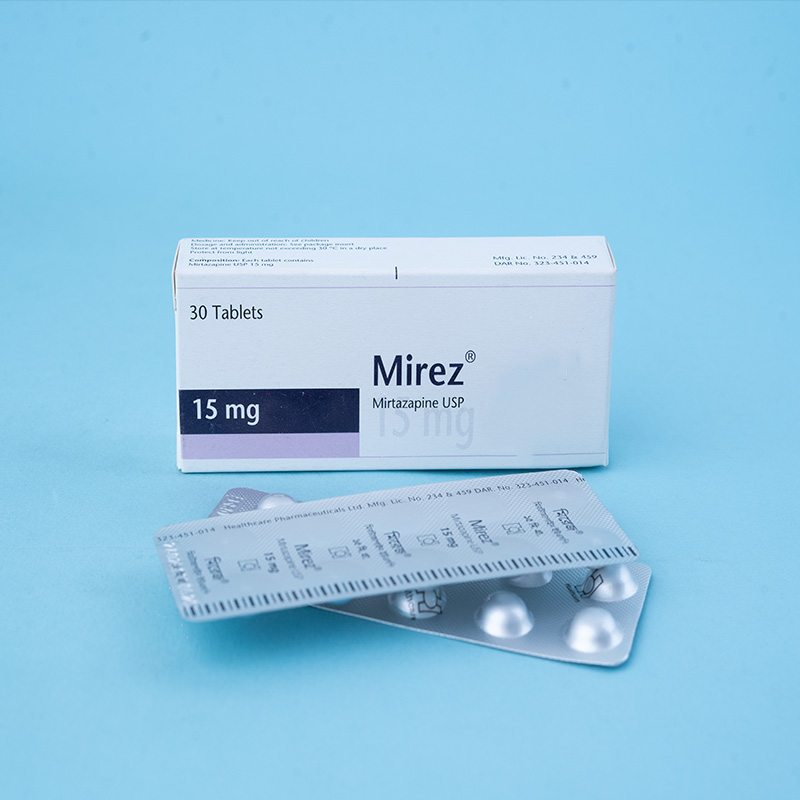
Mirez® 7.5 mg tablets: Each tablet contains Mirtazapine USP 7.5 mg.
Mirez® 15 mg tablets: Each tablet contains Mirtazapine USP 15 mg.
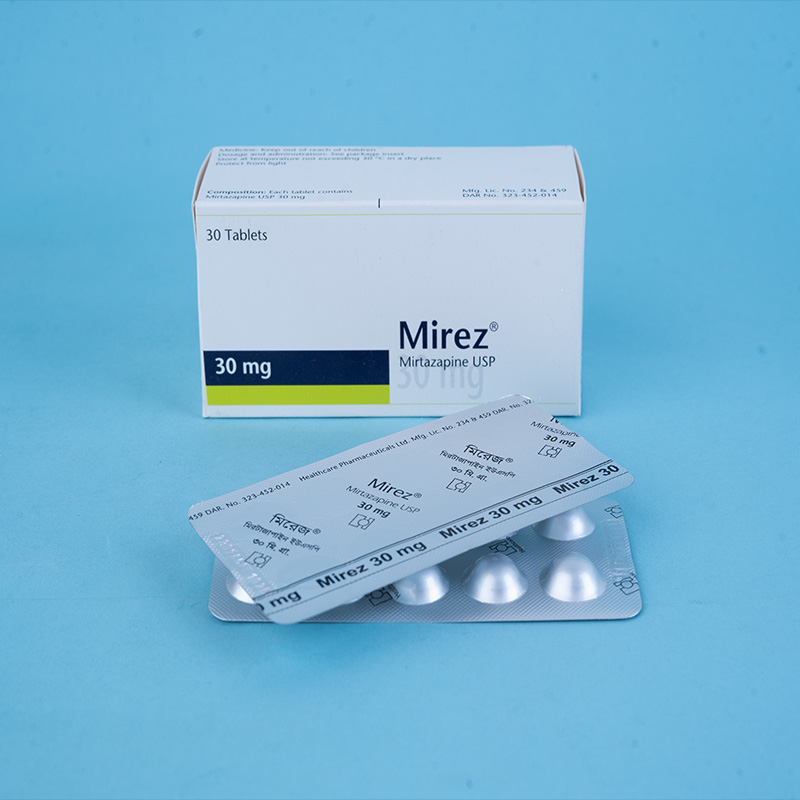
Mirez® 30 mg tablets: Each tablet contains Mirtazapine USP 30 mg.
Mirez® (Mirtazapine) is a tetracyclic antidepressant belonging to the piperazino-azepine group of compounds. Mirtazapine acts as an antagonist of central alpha-2 autoreceptors and hetero-adrenoceptors, increasing the release of noradrenaline and serotonin. The released serotonin acts specifically via 5-HT1 receptors as 5-HT2 and 5-HT3 receptors are selectively blocked by mirtazapine. Mirtazapine is a moderate antagonist of muscarinic receptors, which explains the relatively low incidence of anticholinergic side effects associated with its use.
After oral administration of Mirez® tablets, mirtazapine is rapidly and well-absorbed, reaching peak plasma levels in about 2 hours. Mirtazapine is approximately 85% bound to plasma proteins. The mean elimination half-life is 20-40 hours (26 hours in males and 37 hours in females), allowing for once-daily dosing. Mirtazapine follows linear pharmacokinetics within the recommended dose range and is extensively metabolized. It is eliminated via urine and feces within four days. Major pathways of biotransformation include demethylation and oxidation followed by conjugation.
Mirez® is indicated for the treatment of:
Major depressive disorder
Depression with anxiety
Depression with insomnia
The recommended starting dose for Mirez® tablets is 15 mg/day, administered as a single dose, preferably in the evening or before sleep. The effective dose range is 15-45 mg/day. Patients not responding to the initial 15 mg dose may benefit from dose increases to 30 mg or up to a maximum of 45 mg/day. Due to the elimination half-life of 20-40 hours, dose adjustments should be made at intervals of 1-2 weeks to allow sufficient time for a therapeutic response. Mirez® is not recommended for use in children.
If a dose is missed, it should be taken as soon as remembered unless it is close to the next scheduled dose. If it is almost time for the next dose, skip the missed dose and continue with the regular dosing schedule. Do not take a double dose or more than the prescribed dose.
Mirez® is contraindicated in patients with hypersensitivity to mirtazapine or any of its excipients. It should not be used concomitantly with monoamine oxidase inhibitors (MAOIs).
Commonly reported side effects include increased appetite, weight gain, sedation, edema, dizziness, headache, and, less commonly, postural hypotension and abnormal dreams. Rarely, side effects such as mania, convulsions, tremor, myoclonus, paraesthesia, arthralgia, myalgia, restless legs, and reversible agranulocytosis may occur.
Exercise caution in patients with epilepsy, hepatic or renal impairment, cardiac disorders, hypotension, history of urinary retention, angle-closure glaucoma, diabetes mellitus, psychosis (may aggravate symptoms), and bipolar depression. Avoid abrupt withdrawal of Mirez®.
Mirtazapine should not be used within two weeks of discontinuing MAOIs, and at least two weeks should elapse after stopping MAOIs before initiating mirtazapine therapy. Concurrent use with alcohol, anxiolytics, or hypnotics may potentiate sedative effects.
There are no adequate and well-controlled studies in pregnant women or nursing mothers. Mirez® should be avoided during pregnancy and breastfeeding unless the potential benefits outweigh the risks.
Overdose symptoms include central nervous system depression, disorientation, prolonged sedation, tachycardia, and mild hyper- or hypotension. Management includes gastric lavage and appropriate symptomatic and supportive therapy.
Mirez® 7.5 mg tablets: Each box contains 3 x 10 tablets in Alu-Alu blister packs.
Mirez® 15 mg tablets: Each box contains 3 x 10 tablets in Alu-Alu blister packs.
Mirez® 30 mg tablets: Each box contains 5 x 6 tablets in Alu-Alu blister packs.
Store in a dry place at a temperature not exceeding 30°C. Protect from light.
Medicine: Keep out of reach of children.