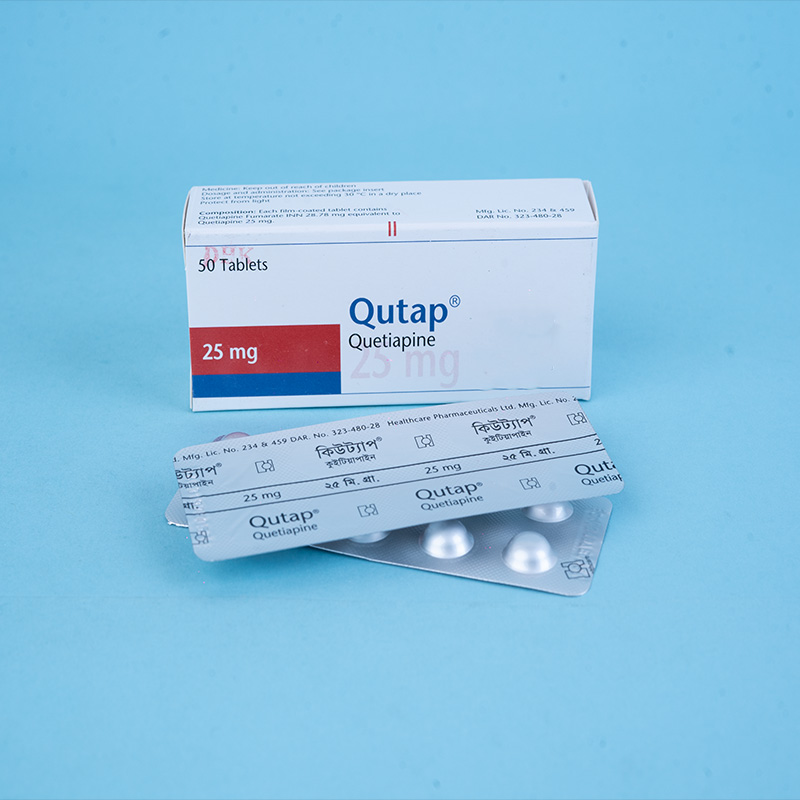
Qutap® 25 mg: Each film-coated tablet contains Quetiapine Fumarate INN 28.78 mg, equivalent to Quetiapine 25 mg.
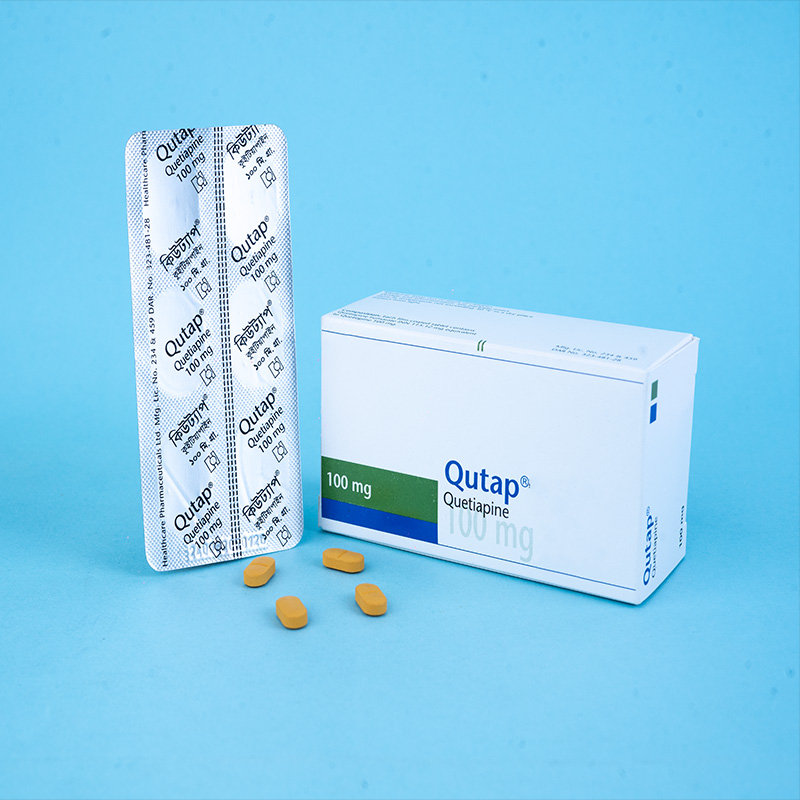
Qutap® 100 mg: Each film-coated tablet contains Quetiapine Fumarate INN 115.12 mg, equivalent to Quetiapine 100 mg.
Quetiapine is an atypical antipsychotic agent that interacts with a broad range of neurotransmitter receptors. It exhibits a higher affinity for serotonin (5HT2) receptors in the brain than for dopamine D1 and D2 receptors. Quetiapine also has a high affinity for histaminergic and adrenergic-1 receptors, a lower affinity for adrenergic-2 receptors, and no appreciable affinity for cholinergic muscarinic or benzodiazepine receptors. Quetiapine is active in tests for antipsychotic activity, such as conditioned avoidance.
Treatment of schizophrenia.
Treatment of manic episodes associated with bipolar disorder.
General Instructions:
Qutap® should be administered twice daily, with or without food.
Adults:
Schizophrenia:
The total daily dose for the first four days of therapy:
Day 1: 50 mg
Day 2: 100 mg
Day 3: 200 mg
Day 4: 300 mg
From Day 4 onwards, the dose should be titrated to the usual effective dose range of 300 to 450 mg/day. Depending on clinical response and tolerability, the dose may be adjusted within the range of 150 to 750 mg/day.
Manic Episodes Associated with Bipolar Disorder:
As monotherapy or adjunct therapy with mood stabilizers:
Day 1: 100 mg
Day 2: 200 mg
Day 3: 300 mg
Day 4: 400 mg
Further dosage adjustments up to 800 mg/day by Day 6 should be made in increments of no greater than 200 mg/day. The dose may be adjusted based on clinical response and tolerability, within the range of 200 to 800 mg/day. The usual effective dose is 400 to 800 mg/day.
Elderly:
Qutap® should be used with caution in elderly patients, especially during initial dosing. Start with 25 mg/day and increase daily in increments of 25 to 50 mg until an effective dose is reached, which is generally lower than in younger patients.
Qutap® is contraindicated in patients hypersensitive to any of the product's components.
Use with caution in patients with cardiovascular disease, cerebrovascular disease, or conditions predisposing to hypotension.
May induce orthostatic hypotension, especially during the initial dose-titration period, which is more common in elderly patients.
Although not associated with a persistent increase in QTc interval in clinical trials, caution is advised when prescribing with drugs known to prolong the QTc interval, particularly in elderly patients.
The safety and efficacy of Qutap® during pregnancy have not been established. Use only if the benefits justify the potential risks.
The degree of excretion of quetiapine in human milk is unknown. Breastfeeding should be avoided while taking Qutap®.
Commonly reported adverse drug reactions include:
Somnolence, dizziness, dry mouth, mild asthenia, constipation, tachycardia, orthostatic hypotension, and dyspepsia.
Rarely, it may cause syncope, neuroleptic malignant syndrome, leucopenia, neutropenia, and peripheral edema.
Caution is advised when combining Qutap® with other centrally acting drugs and alcohol.
The pharmacokinetics of lithium and valproic acid are not clinically significantly altered when co-administered with Qutap®.
Co-administration with thioridazine increases the clearance of quetiapine.
Symptoms of overdose include drowsiness, sedation, tachycardia, and hypotension. There is no specific antidote for quetiapine overdose. In cases of severe intoxication, intensive care procedures such as maintaining a patent airway, oxygenation, ventilation, and cardiovascular monitoring are recommended.
Qutap® 25 mg: Each box contains 5 x 10 tablets in Alu-Alu blister packs.
Qutap® 100 mg: Each box contains 5 x 6 tablets in Alu-Alu blister packs.
Store in a dry place at a temperature not exceeding 30°C.
Protect from light.
Keep out of reach of children.