Residon® 1 mg tablets: Each tablet contains Risperidone USP 1 mg.
Residon® 2 mg tablets: Each tablet contains Risperidone USP 2 mg.
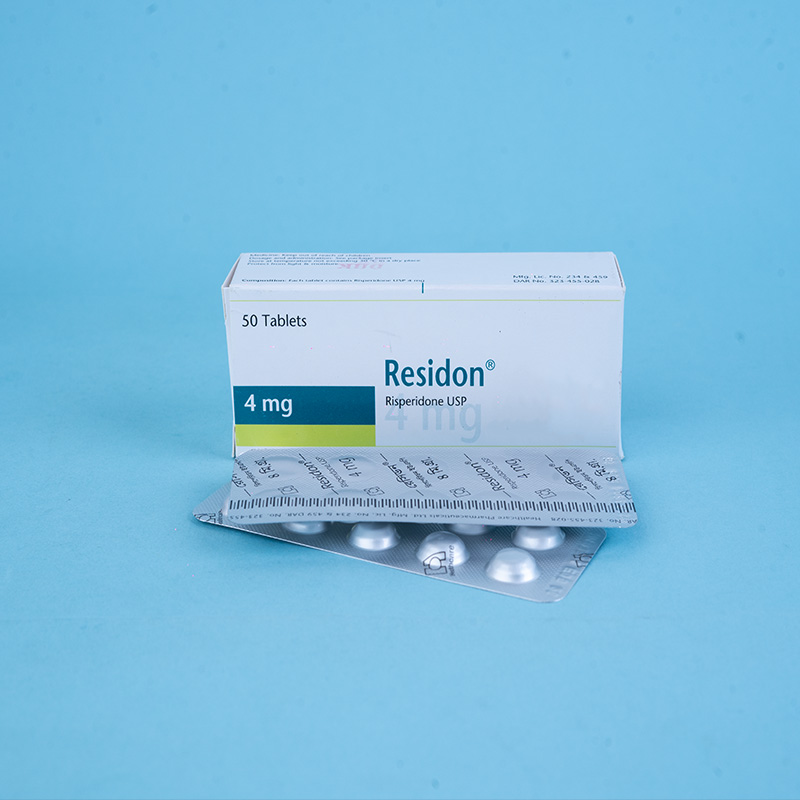
Residon® 4 mg tablets: Each tablet contains Risperidone USP 4 mg.
Risperidone is a benzisoxazole derivative and an antipsychotic agent. Its antipsychotic activity is attributed to its antagonist effects on dopamine (D2 subtype) and serotonin (5HT2 subtype) receptors. Additionally, it is an antagonist of alpha1, alpha2, and H1 receptor subtypes, which mediate the drug's non-antipsychotic effects.

Risperidone has shown efficacy in reducing hallucinatory behavior, paranoia, bizarre thought content, and conceptual disorganization during short-term treatment of schizophrenia. Orthostatic hypotension may occur during therapy initiation due to alpha-adrenergic receptor antagonism.

Absorption after oral administration is rapid, with up to 85% of the dose absorbed.
Absolute oral bioavailability is approximately 70%.
Plasma protein binding is about 90%.
Metabolized extensively by the hepatic microsomal CYP450IID6 enzyme into 9-hydroxy-risperidone, an active metabolite.
Tmax: 1 to 3 hours.
Half-life of elimination: 3 to 20 hours for the parent drug and 21 to 30 hours for the metabolite.
Residon® is indicated for managing manifestations of psychotic disorders, including:
Acute schizophrenia
Chronic schizophrenia
Affective symptomatology of schizophrenia
Other psychoses
Adults:
Start with 1 mg twice daily on Day 1, 2 mg twice daily on Day 2, and 3 mg twice daily on Day 3. Stabilize the dose for one week. Adjust further in 1 mg increments weekly, up to a maximum dose of 8 mg/day. The usual effective dose is 4–6 mg/day. Discontinue if tardive dyskinesia occurs.
Elderly:
Start with 0.5 mg twice daily on Day 1. Increase by 0.5 mg daily to reach 1–2 mg twice daily. Monitor closely, especially in the presence of hepatic or renal impairment, as these patients are prone to profound hypotension.
Hypersensitivity to risperidone.
Common: Extrapyramidal symptoms (at doses above 6 mg/day), tremor, rigidity, hypokinesia, dystonia, dizziness, constipation, anxiety, somnolence, rash, rhinitis, and tachycardia.
Rare: Arthralgia, aggressive reactions, and visual disturbances.
Use with caution in conditions like acute myocardial infarction (AMI), ischemic heart disease (IHD), heart failure, conduction abnormalities, dehydration, or hypovolemia.
Monitor patients with epilepsy, hyperprolactinemia, parkinsonism, renal or hepatic impairment, and the elderly closely.
Monitor for neuroleptic malignant syndrome and tardive dyskinesia, and discontinue risperidone if these occur.
Risperidone may prolong the QT interval. Though no confirmed cases of proarrhythmic effects have been reported, caution is advised.
Caution when used with CNS-active drugs, levodopa, antihypertensive agents, alcohol, carbamazepine (increases risperidone clearance), or clozapine (decreases risperidone clearance).
Adequate and well-controlled studies are lacking. Use only if the potential benefits justify the potential risks to the fetus.
Safety in children under 15 years has not been established.
Clinical experience with overdose is limited, and no fatalities have been reported.
Management includes establishing an airway, gastric lavage, activated charcoal administration, and continuous ECG monitoring for arrhythmias. No specific antidote is available; supportive measures are the mainstay of therapy.
Residon® 1 mg: Each box contains 5×10 tablets in an alu-alu blister pack.
Residon® 2 mg: Each box contains 5×10 tablets in an alu-alu blister pack.
Residon® 4 mg: Each box contains 5×10 tablets in an alu-alu blister pack.

Store at a temperature not exceeding 30°C in a dry place.
Protect from light and moisture.
Keep out of reach of children.