Escitalopram is an oral drug used for treating depression and generalized anxiety disorder. It works by affecting neurotransmitters in the brain, the chemical messengers that nerves use to communicate with one another. Neurotransmitters are produced and released by nerves, then travel to nearby nerves where they attach to receptors. Some neurotransmitters that are released do not bind to receptors and are reabsorbed by the nerves that produced them. This process is referred to as "reuptake."
Many experts believe that an imbalance of neurotransmitters causes depression. Escitalopram prevents the reuptake of one neurotransmitter, serotonin, by nerves, which increases the amount of serotonin available in the brain to attach to receptors. Chemically, escitalopram is very similar to citalopram. Both drugs belong to the class of medications called selective serotonin reuptake inhibitors (SSRIs).
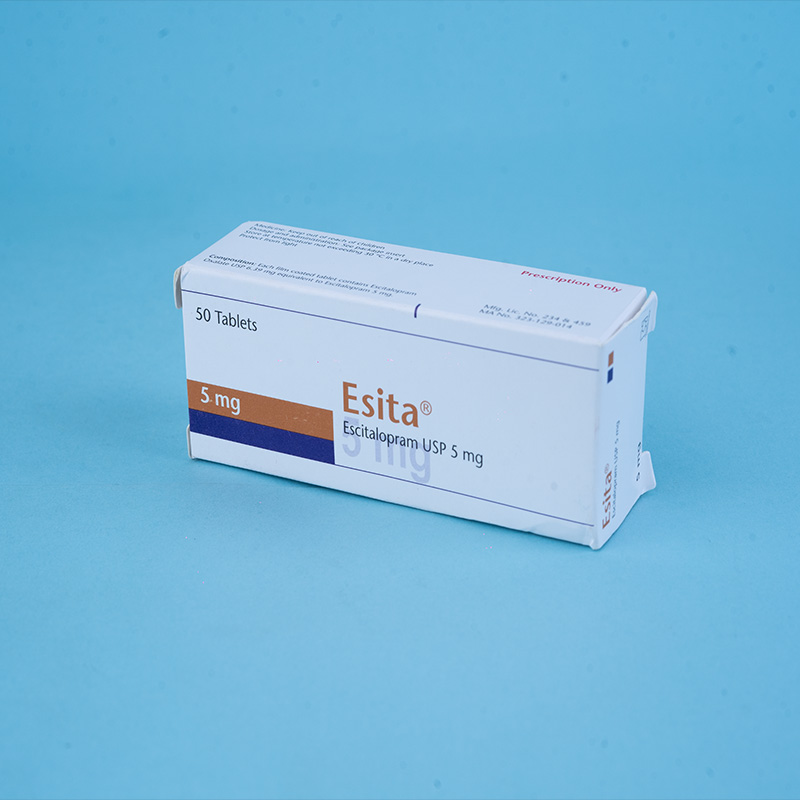
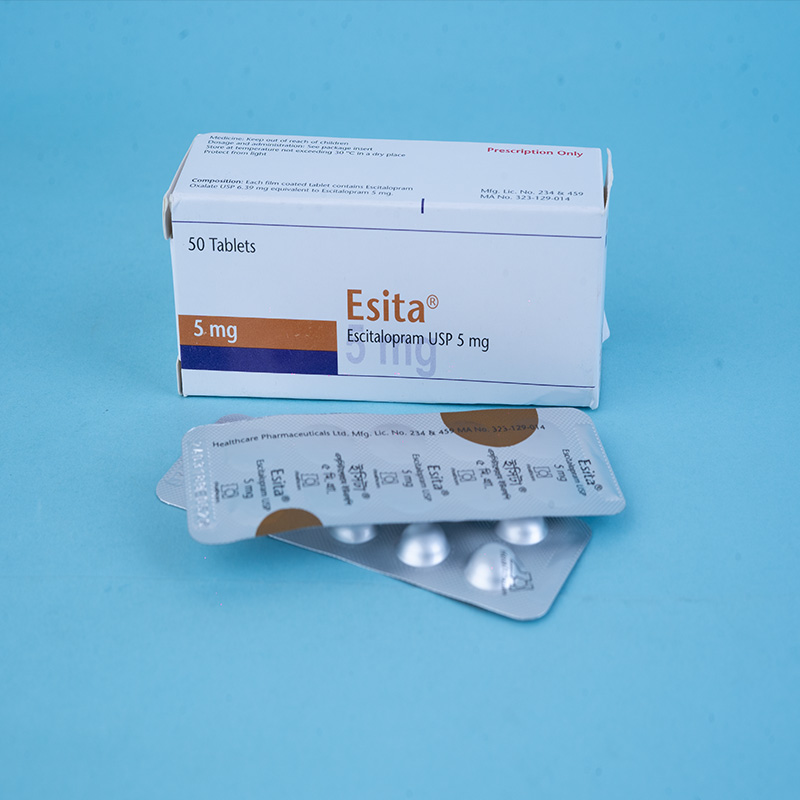
Esita® 5 mg tablet: Each film-coated tablet contains Escitalopram Oxalate INN equivalent to Escitalopram 5 mg.
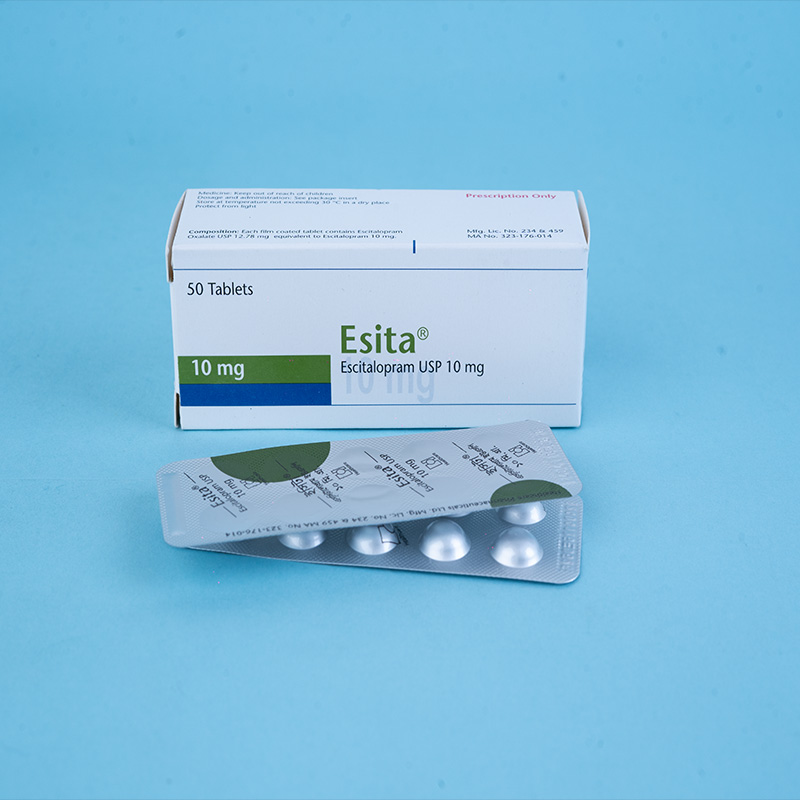
Esita® 10 mg tablet: Each film-coated tablet contains Escitalopram Oxalate INN equivalent to Escitalopram 10 mg.
Escitalopram is approved for the treatment of depression and generalized anxiety disorder. Drugs in the SSRI class have also been studied for use in individuals with obsessive-compulsive disorders and panic disorders.
Concomitant use with monoamine oxidase inhibitors (MAOIs) is contraindicated.
Concomitant use with pimozide is contraindicated.
Escitalopram is contraindicated in patients with hypersensitivity to escitalopram, citalopram, or any inactive ingredients in the formulation.
The most common side effects associated with escitalopram include:
Agitation or restlessness
Blurred vision
Diarrhea
Difficulty sleeping
Drowsiness
Dry mouth
Fever
Frequent urination
Headache
Indigestion
Nausea
Increased or decreased appetite
Increased sweating
Sexual difficulties (e.g., decreased sexual ability or desire, ejaculatory delay)
Taste alterations
Tremor (shaking)
Weight changes
Although sexual difficulties may occur due to depression itself, they can also be a result of the drugs used to treat depression. About one in 11 men given escitalopram report difficulty experiencing ejaculation.
During the use of escitalopram and other SSRIs or SNRIs (serotonin and norepinephrine reuptake inhibitors), there have been reports of adverse events upon abrupt discontinuation of these drugs. Symptoms include:
Dysphoric mood
Irritability
Agitation
Dizziness
Sensory disturbances (e.g., paresthesias such as electric shock sensations)
Anxiety
Confusion
Headache
Lethargy
Emotional lability
Insomnia
Hypomania
While these symptoms are generally self-limiting, serious discontinuation symptoms have been reported. A gradual reduction in dose rather than abrupt cessation is recommended. If intolerable symptoms occur following dose reduction or discontinuation, resuming the previously prescribed dose may be considered. The physician can then decrease the dose at a more gradual rate.

The usual starting dose of escitalopram is 10 mg once daily. Benefits may take up to 4 weeks to become apparent. Escitalopram can be taken with or without food. The recommended doses are similar for both older and younger patients.

All SSRIs, including escitalopram, should not be taken with MAOI-class antidepressants, such as isocarboxazid, phenelzine, tranylcypromine, and procarbazine, as this may lead to confusion, high blood pressure, high fevers, tremor, muscle rigidity, and increased activity.
Similar interactions may occur with selegiline, fenfluramine, and dexfenfluramine.
Tryptophan, when taken with any SSRI, can cause headache, nausea, sweating, and dizziness.
At least 14 days should elapse after discontinuing escitalopram before starting an MAOI.
The safety of escitalopram during pregnancy and lactation has not been established. Escitalopram should only be used during pregnancy if, in the opinion of the physician, the expected benefits to the patient outweigh the potential risks to the fetus.
Escitalopram is excreted in human milk. It should not be given to nursing mothers unless the expected benefits to the patient outweigh the potential risks to the child, as determined by the physician.
Store in a cool, dry place below 30°C. Protect from light.
Keep out of reach of children.