Latuda® 20: Each film-coated tablet contains Lurasidone Hydrochloride INN 20 mg.
Latuda® 40: Each film-coated tablet contains Lurasidone Hydrochloride INN 40 mg.
The mechanism of action of Lurasidone in the treatment of schizophrenia and bipolar depression is unknown. However, its efficacy in schizophrenia and bipolar depression may be mediated through a combination of central dopamine Type 2 (D2) and serotonin Type-2 (5HT2A) receptor antagonism.
Lurasidone is indicated for:
Schizophrenia
Depressive episodes associated with Bipolar disorder
Commonly observed adverse reactions include somnolence, akathisia, extrapyramidal symptoms, and nausea.
Lurasidone should be taken with food (at least 350 calories). Administration with food substantially increases the absorption of Lurasidone.
Schizophrenia
The recommended starting dose of Lurasidone is 40 mg once daily. Initial dose titration is not required. The maximum recommended dose is 160 mg per day.
Depressive Episodes Associated with Bipolar I Disorder
The recommended starting dose of Lurasidone is 20 mg given once daily as monotherapy or as adjunctive therapy with lithium or valproate. Initial dose titration is not required. The maximum recommended dose, as monotherapy or as adjunctive therapy with lithium or valproate, is 120 mg per day.
Lurasidone is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. It is also contraindicated for concomitant use with strong CYP3A4 inhibitors (e.g., ketoconazole) and strong CYP3A4 inducers (e.g., rifampin).
It may cause increased mortality in elderly patients with dementia-related psychosis.
It may increase suicidal thoughts and behaviors in adolescents and young adults.
Pregnancy: Pregnancy Category B.
Nursing Mothers: Lurasidone is excreted in human milk. Due to the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Safety and effectiveness in pediatric patients have not been established.
Lurasidone should not be used concomitantly with strong CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil, etc.) or strong CYP3A4 inducers (e.g., rifampin, phenytoin, carbamazepine, etc.).
An overdose of 560 mg of Lurasidone does not cause any significant reactions.
Store at a temperature not exceeding 30°C in a dry place. Protect from light.
Medicine: Keep out of reach of children.
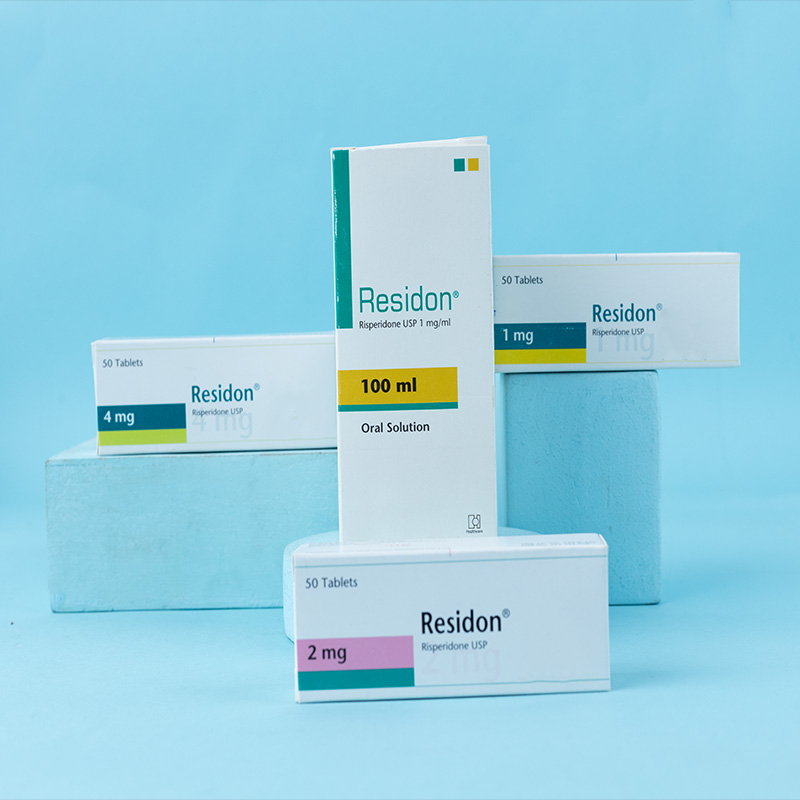
Latuda® 20: Each box contains 30 tablets in an Alu-Alu blister pack.
Latuda® 40: Each box contains 30 tablets in an Alu-Alu blister pack.