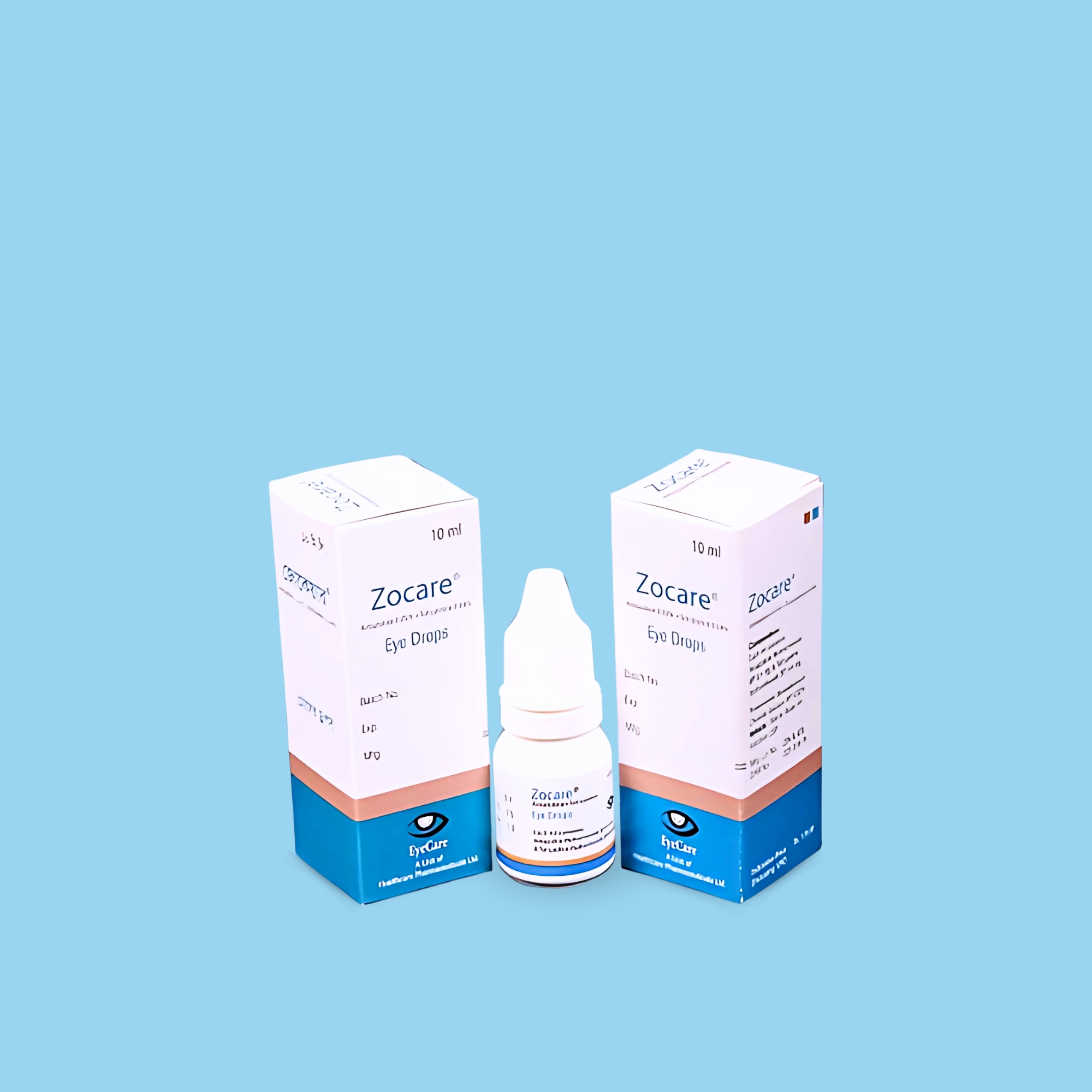
Bludrop® Eye Drops are used for the symptomatic treatment of dry eye conditions, including keratoconjunctivitis sicca. It is also used as a substitute for tear fluid in cases of unstable tear film or insufficient moistening of the eye surface.
1 drop, 4 times daily or as required, depending on the severity of the condition, to be instilled into the conjunctival sac.
Bludrop Eye Drops are contraindicated in patients with known hypersensitivity to any ingredient of the product.
Occasionally, mild transient burning or a sticky sensation may occur, and very rarely, irritation or hypersensitivity reactions have been reported. Blurred vision after application may also occur.
Patients who experience blurred vision after the application of the product should not drive or operate machinery until their vision has cleared.
Contact lenses should not be worn during instillation of the product. After instillation, there should be an interval of at least 30 minutes before reinsertion of contact lenses.
If additional local ocular treatment is being used, such as glaucoma therapy, there should be an application interval of at least 5 minutes between the two medications. Bludrop should always be the last medication instilled.
There is no experience regarding the safety of Povidone Eye Drops during pregnancy or lactation. Therefore, administration during pregnancy and lactation is not recommended, except for compelling reasons.
High concentrations of Sodium Sulphate in cold conditions and Sodium Chloride in warm conditions can result in precipitation of Povidone. Depending on the ionic strength of the solution, Methylparaben and Propylparaben can easily form complexes with Povidone.
The drug should be used within 30 days after first opening. Store at 15°-25°C. Protect from light. The bottle should be closed tightly immediately after use.
Medicine: Keep out of reach of children.