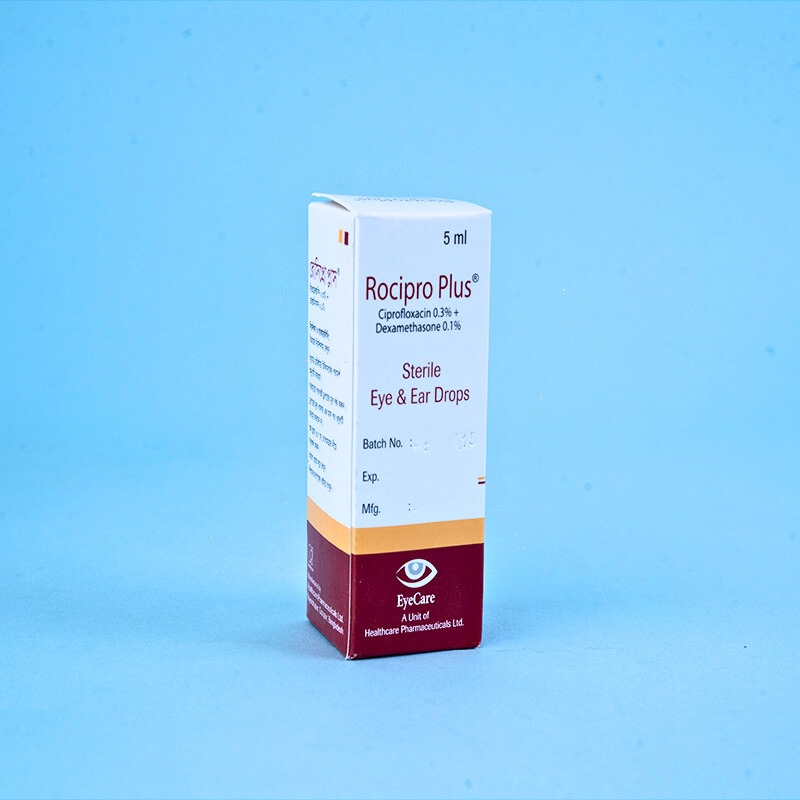
Ciprofloxacin is a member of the fluoroquinolone class, which exhibits antibacterial activity by inhibiting DNA gyrase and topoisomerase IV in bacteria. Dexamethasone, a potent corticosteroid, suppresses the inflammatory response to chemical, immunological, or mechanical irritants.

Eye: Rocipro Plus® is indicated for the treatment of steroid-responsive inflammatory ocular conditions where bacterial infections or the risk of bacterial infections coexist. The combination can also be used for post-operative inflammation and other ocular inflammations associated with infection.
Ear: Rocipro Plus® is also indicated for the treatment of ear infections accompanied by inflammation, such as otitis externa and acute or chronic otitis media. The combination may also be used post-operatively in the ear.
For Eye: Instill 1 drop into the conjunctival sac(s) every four to six hours. During the initial 24 to 48 hours, the dosage may be increased to 1 drop every two hours.
For Ear:Acute otitis media in pediatric patients with tympanostomy tubes: Instill 4 drops into the affected ear twice daily for 7 days.
Acute otitis externa: Instill 4 drops into the affected ear twice daily for 7 days.
Rocipro Plus® is contraindicated in patients with hypersensitivity to any of its ingredients. It should not be used in cases of herpes simplex and other viral conditions, mycosis, glaucoma, newborn infants, or fungal diseases of ocular or auricular structures.
The possibility of fungal infections of the cornea should be considered after long-term steroid use and in patients with persistent corneal ulceration. Prolonged use of antibiotics may result in the overgrowth of non-susceptible organisms.
Prolonged use of topical ophthalmic steroids may lead to glaucoma, resulting in optic nerve damage, defects in visual acuity and visual fields, and posterior subcapsular cataract formation. Intraocular pressure should be routinely monitored.
Eye: The most frequently reported adverse reactions associated with Ciprofloxacin include transient ocular burning or discomfort. Other reported reactions include stinging, redness, itching, conjunctivitis/keratitis, periocular/facial edema, foreign body sensation, photophobia, blurred vision, tearing, dryness, and eye pain. Rare reports of dizziness have also been received. Adverse effects due to the steroid component include elevated intraocular pressure (IOP), which may lead to glaucoma, infrequent optic nerve damage, posterior subcapsular cataract formation, and delayed wound healing.
Ear: The most common adverse effects include discomfort and ear pain. Other reported reactions include irritability, dizziness, and erythema.
Pregnancy: There are no adequate and well-controlled studies in pregnant women. Rocipro Plus® Sterile Eye & Ear Drops should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Therefore, caution should be exercised when administering Rocipro Plus® Sterile Eye & Ear Drops to a nursing mother.
Pediatric Use: The safety and effectiveness of this product in pediatric patients below 1 year of age have not been established.
The drug should be used within 30 days after first opening. Store at a temperature not exceeding 30°C in a dry place. Protect from light. Close the bottle tightly immediately after use.
Medicine: Keep out of reach of children.