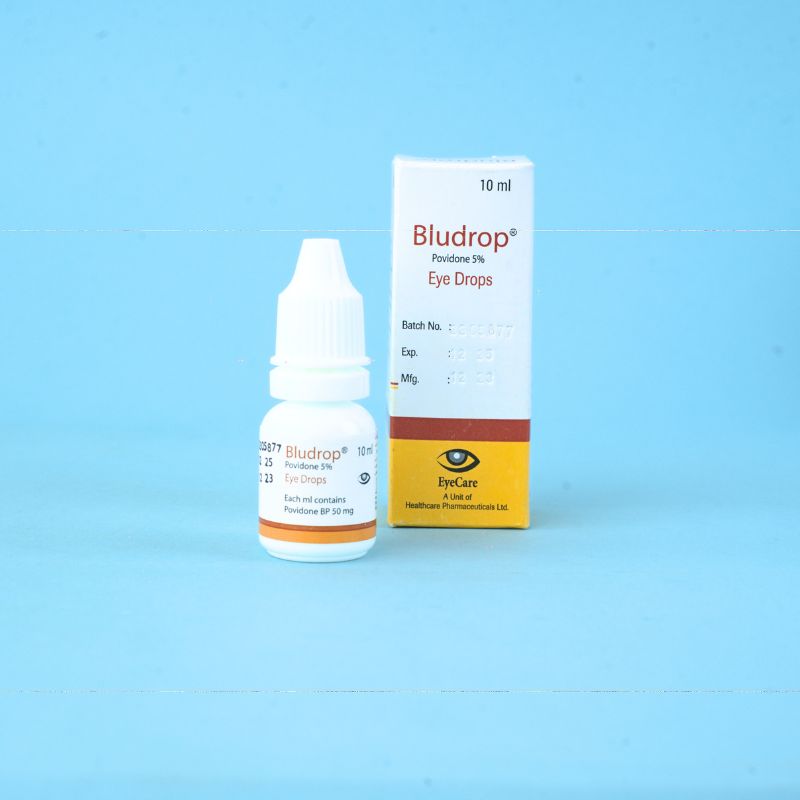
Cream or ointment:
Therapeutically: As an adjunct to systemic therapy in the following indications-
- Primary or secondary topical infections
- Infected surgical incisions
- Infected decubitus or stasis ulcers
- Pyodermas
- Secondarily infected dermatoses
- Infected traumatic lesions
- Prophylactically:
- To prevent microbial contaminations in burns, incisions and other topical lesions
- For degerming skin in hyperalimentation, the umbilical area or circumcision
- Its use for abrasions, minor cuts, and wounds prevents the development of infections and permits wound healing.
Povidone Iodine is a complex of iodine and an organic polymer, povidone. This polymerization makes Povidone Iodine superior to ordinary elemental iodine. It prolongs the germicidal activity of iodine by liberating elemental iodine slowly. Consequently it has a lower toxicity than elemental iodine. It gives rapid microbicidal activity against both Gram-positive and Gram-negative bacteria, protozoa, viruses and fungi/yeasts. It is also sporicidal. It is the only microbicide with this broad spectrum of activity. It is non-staining, exerts prolong germicidal action and is also active in the presence of soap, blood, serum, pus, mucosal secretions and water.

1 drop 4 times daily or as required, depending upon the severity of the disease, to be instilled into the conjunctival sac

It exhibits interaction with strong alkali, sodium thiosulphate, sodium metabisulphite and thiomersal. Use with concurrent lithium therapy has been shown to exhibithypothyroidic effect.
It can cause hypersensitivity reactions. Regular use in patients with thyroid disorders (in particular nodular colloid goitre, endemic goitre and Hashimoto's thyroiditis) or those receiving lithium therapy is to be avoided. In severely burnt patients serum iodide levels should be assessed due to possible hepatic and renal impairment. Povidone Iodine Powder should not be used in serious cavities and in Children under the age of 2 years.
Bludrop may cause hypersensitivity reactions and irritation of the skin and mucous membranes. The application of Bludrop to severe burns or to large areas otherwise denuded of skin may produce systemic adverse effects such as metabolic acidosis, hypernatraemia, and impairment of renal function. It may interfere with thyroid function tests.
Povidone Iodine Cream, Solution & Powder are not recommended for use during pregnancy because of the possibility of absorption of sufficient iodine to affect the fetal thyroid. American Academy of Pediatrics considers that the use of Povidone-Iodine is usually compatible with breast feeding. Consult Registered Physician. Regular use of Gargle & Mouthwash and Surgical Scrub should also be avoided in pregnant and lactating women.
There is no experience regarding the safety of the Povidone eye drops in human pregnancy or lactation. Administration during pregnancy and lactation is therefore not recommended, except for compelling reasons.