Moxifloxacin shows antibacterial activity by inhibiting the bacterial enzyme topoisomerase II (DNA gyrase) and topoisomerase IV.
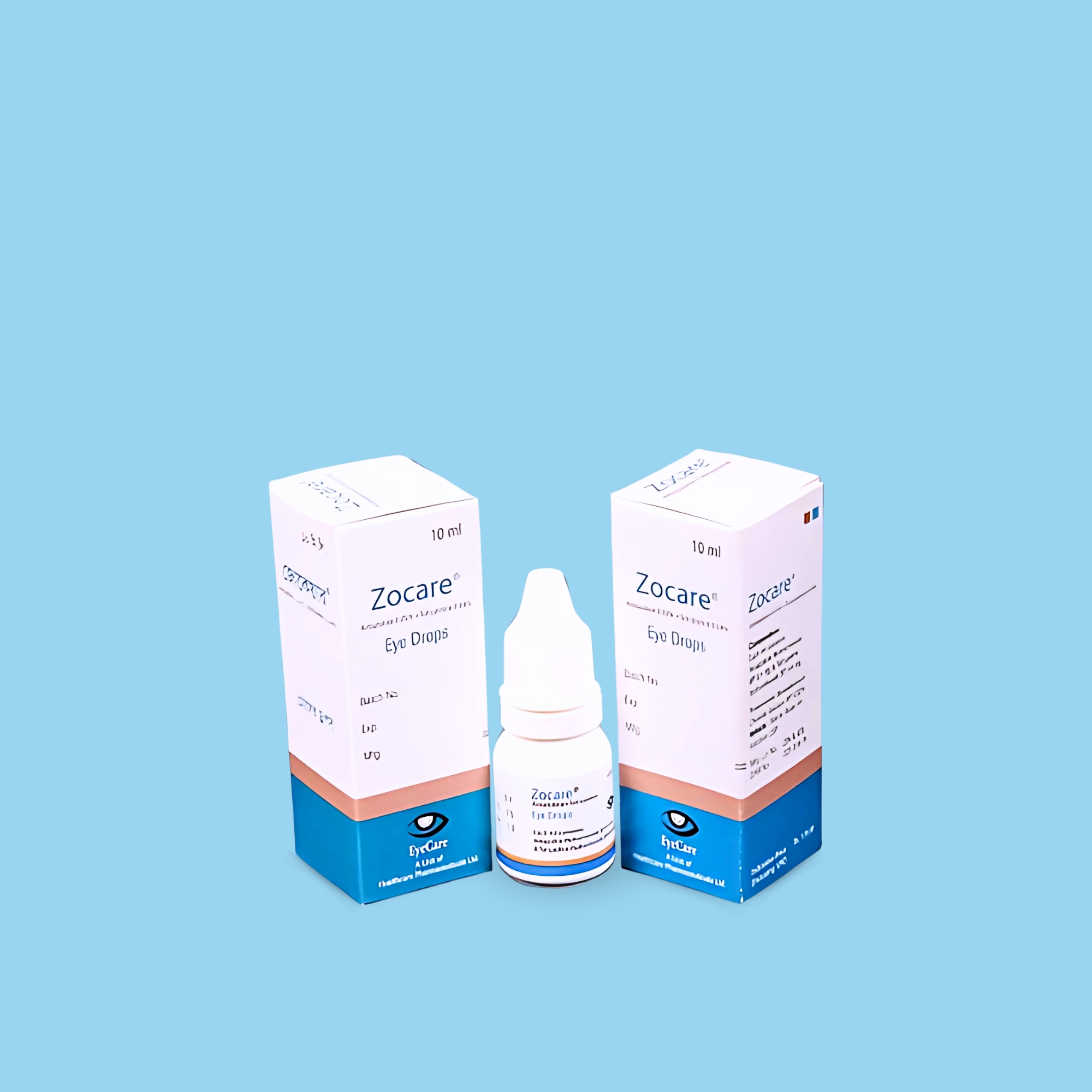
Moxivin® 0.5% Eye Drops is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms:
Aerobic gram-positive microorganisms: Corynebacterium species, Micrococcus luteus, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus warneri, Streptococcus pneumoniae, Streptococcus viridans group.
Aerobic Gram-negative microorganisms: Acinetobacter iwoffii, Haemophilus influenzae, Haemophilus parainfluenzae.
One drop in the affected eye, 3 times per day for 7 days.
Moxifloxacin Hydrochloride eye drops is contraindicated in patients with a history of hypersensitivity to Moxifloxacin, other quinolones, or any of the components in the medication.
The most frequently reported ocular side effects were conjunctivitis, decreased visual acuity, dry eye, keratitis, ocular discomfort, ocular hyperemia, ocular pain, ocular pruritus, subconjunctival hemorrhage, and tearing. These events occurred in approximately 1-6% of patients.
Prolonged use may result in the overgrowth of non-susceptible organisms, including fungi, as with other anti-infectives.
Moxifloxacin eye drops should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus, as there are no adequate and well-documented studies in pregnant women.
Moxifloxacin has not been measured in human milk, although it can be presumed to be excreted in human milk. Caution should be exercised when Moxifloxacin Hydrochloride eye drops is administered to a lactating mother.
The safety and effectiveness of Moxifloxacin eye drops in infants below 1 year of age have not been established. However, clinical studies have shown that Moxifloxacin eye drops can be used in children younger than 1 month of age.
No overall differences in safety and effectiveness have been observed between elderly and younger patients.
Drug-drug interaction studies have not been conducted with Moxifloxacin Hydrochloride eye drops. In vitro studies indicate that Moxifloxacin does not inhibit CYP3A4, CYP2D6, CYP2C9, CYP2C19, or CYP1A2, indicating that Moxifloxacin is unlikely to alter the pharmacokinetics of drugs metabolized by these cytochrome P450 isoenzymes.
The drug is to be used within 30 days after first opening. Store at 15°-25°C.
Protect from light.The bottle should be closed tightly immediately after use
Medicine: Keep out of reach of children.