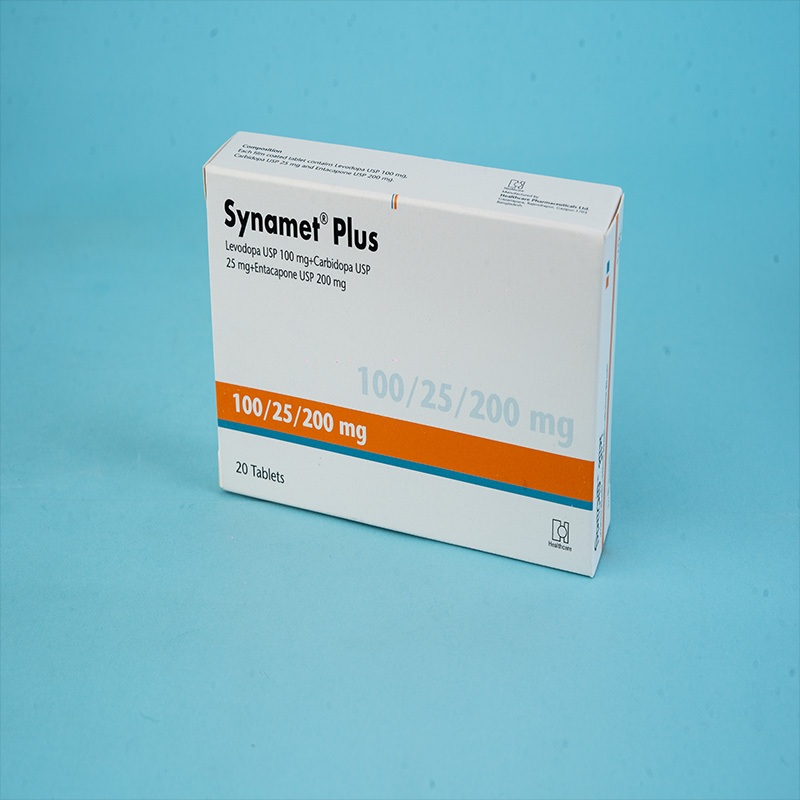
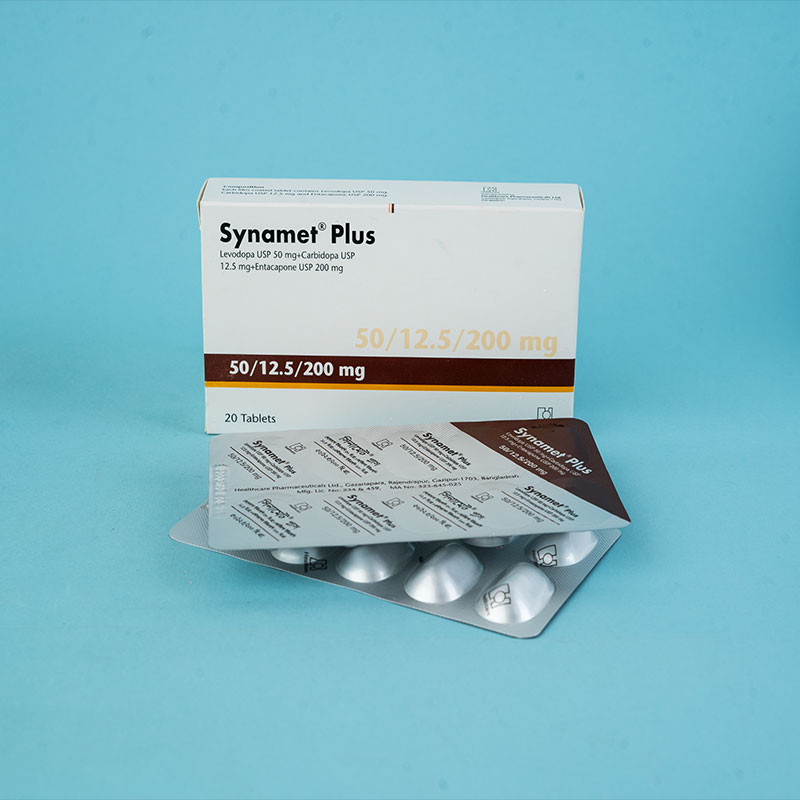
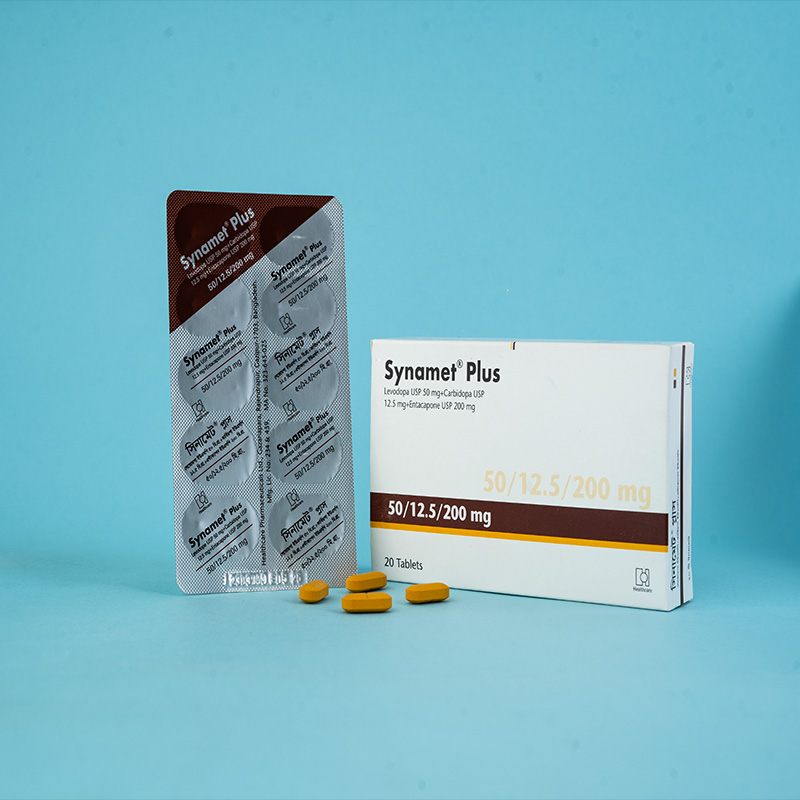
Stalevo® is indicated for the treatment of adult patients with Parkinson's disease who experience end-of-dose motor fluctuations and are not stabilized on levodopa/dopa decarboxylase (DDC) inhibitor treatment.
Stalevo® is a combination of levodopa, carbidopa, and entacapone for the treatment of Parkinson's disease.
Levodopa is a metabolic precursor of dopamine that crosses the blood-brain barrier and is converted into dopamine.
Carbidopa is a DDC inhibitor that reduces peripheral metabolism of levodopa to dopamine, ensuring more levodopa is available to the brain.
Entacapone is a selective inhibitor of catechol-O-methyltransferase (COMT). When taken together, entacapone enhances plasma levodopa levels, improving control over Parkinson's disease symptoms.

Adults:
The optimal daily dose is determined by careful titration of levodopa in each patient.
Patients should take 1 tablet per dose.
The maximum recommended daily dose of entacapone is 2,000 mg.
It is commonly used for patients already treated with corresponding doses of standard-release levodopa/DDC inhibitors and entacapone.
Method of administration:
The tablet should be taken orally with or without food.
Each tablet contains a single treatment dose and must be taken whole.
Switching from other treatments:
Switching from levodopa/DDC inhibitor and entacapone:
Patients on corresponding doses of levodopa/carbidopa and entacapone can switch directly to the same dose of Stalevo (e.g., 100/25 mg of levodopa/carbidopa with entacapone 200 mg).
For different dosing combinations, careful titration is required to optimize the clinical response.
Switching from levodopa/DDC inhibitor alone:
For patients not on entacapone, initiate Stalevo cautiously, considering their existing levodopa dose.
If the patient experiences dyskinesia or takes more than 800 mg of levodopa daily, initiate entacapone separately before switching to Stalevo.
Dose adjustments:
If more levodopa is needed, increase frequency of doses or switch to a higher-strength Stalevo tablet.
If less levodopa is required, decrease the frequency, extend the time between doses, or switch to a lower-strength tablet.
Discontinuation: Stalevo is discontinued, adjust the dosing of other antiparkinsonian treatments, especially levodopa, to manage symptoms effectively.
Antihypertensive medications: Adding levodopa to patients on antihypertensive drugs may cause symptomatic postural hypotension. Adjusting the antihypertensive dose may be required.
Dopamine receptor antagonists (e.g., antipsychotics, antiemetics), phenytoin, and papaverine may reduce the effectiveness of levodopa.
High protein diets can impair the absorption of levodopa, carbidopa, and entacapone
Stalevo® is contraindicated in patients with:
Severe hepatic impairment
Narrow-angle glaucoma
Pheochromocytoma
Concurrent use with non-selective MAO inhibitors (e.g., phenelzine, tranylcypromine)
A history of Neuroleptic Malignant Syndrome (NMS) and/or nontraumatic rhabdomyolysis
Known hypersensitivity to any of the active ingredients or excipients
Common side effects include: Dyskinesia, nausea, hyperkinesia, change in urine color, diarrhea, and stomach pain.
Other possible side effects: Severe diarrhea, colitis, hallucinations, mental disturbances, orthostatic hypotension, rhabdomyolysis, Neuroleptic Malignant Syndrome (NMS) symptoms, fibrosis, skin cancer.
Pregnancy: Category C. Stalevo® should only be used during pregnancy if the benefits justify the risks to the fetus.
Lactation: Stalevo® should not be used by breastfeeding women due to unknown safety in infants.
Precautions & Warnings: Caution is required when driving or operating machinery due to possible dizziness and orthostatic hypotension.
Regular evaluations of hepatic, hematopoietic, cardiovascular, and renal function are recommended during long-term therapy.
Children: Safety and effectiveness have not been established in pediatric patients.
Elderly: No dose adjustment is required for elderly patients.
Hepatic impairment: Administer cautiously to patients with mild to moderate hepatic impairment. Dose reduction may be necessary.
Renal impairment: Use cautiously in patients with severe renal impairment, including those on dialysis.
Signs of overdose may include:
Agitation, confusion, coma, bradycardia, ventricular tachycardia, Cheyne-Stokes respiration, skin discoloration, chromaturia.
Management: Hospitalization is recommended. General supportive measures, including gastric lavage and charcoal, should be employed.
Store below 30°C, away from light and moisture.
Keep out of the reach of children.