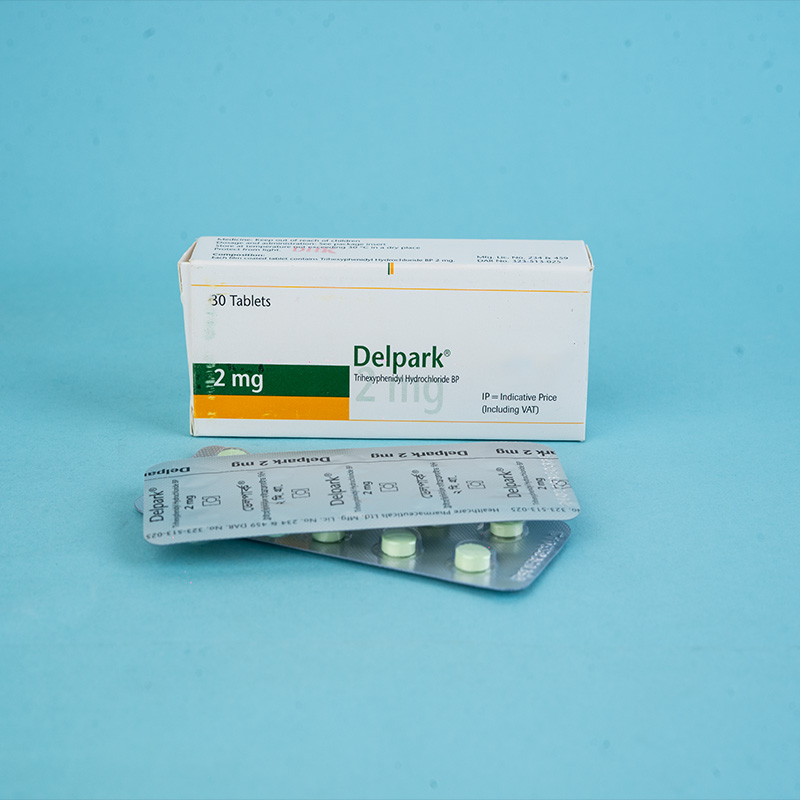
Delpark® (Trihexyphenidyl HCI) is indicated as an adjunct in the treatment of all forms of parkinsonism, including postencephalitic, arteriosclerotic, and idiopathic. It is often used as adjuvant therapy alongside levodopa for these conditions. Additionally, it is indicated for controlling extrapyramidal disorders caused by central nervous system drugs such as dibenzoxazepines, phenothiazines, thioxanthenes, and butyrophenones.
Dosage should be individualized. Therapy should begin with a low dose and gradually increase, especially in patients over 60 years of age. For idiopathic Parkinsonism, the initial dose is 1 mg on the first day, with subsequent increases of 2 mg at intervals of three to five days until a total daily dose of 6-10 mg is reached. Some patients, particularly postencephalitic cases, may require a daily dose of 12-15 mg. For drug-induced Parkinsonism, the daily dosage typically ranges between 5-15 mg, with an initial dose of 1 mg that can be adjusted based on response.
When used with levodopa, the dosage of both drugs may need adjustment, with 3-6 mg of Trihexyphenidyl HCI daily in divided doses usually being sufficient. For concomitant use with other parasympathetic inhibitors, partial substitution is recommended initially, followed by gradual dose adjustments. High doses of Trihexyphenidyl HCI (>10 mg daily) should be divided into three doses at mealtimes and a fourth dose at bedtime.
Trihexyphenidyl HCI is contraindicated in patients with hypersensitivity to the drug and those with narrow-angle glaucoma, as blindness due to long-term use has been reported. Patients should undergo gonioscope evaluation before starting therapy and have close monitoring of intraocular pressure during treatment. Use with caution in hot weather, particularly in chronically ill patients, alcoholics, and those performing manual labor in hot environments, as the drug may impair perspiration, leading to anhidrosis and hyperthermia. Abrupt withdrawal of therapy may result in Neuroleptic Malignant Syndrome (NMS) or exacerbation of Parkinsonism symptoms.
There is insufficient data on the use of Trihexyphenidyl HCI during pregnancy or lactation. It should only be used when the benefits outweigh the risks. Its effects on lactation are not well understood, and it may suppress milk production. Safety and effectiveness in pediatric patients have not been established.
Cannabinoids, barbiturates, opiates, and alcohol may enhance sedative effects when used with Trihexyphenidyl HCI. Concurrent use of monoamine oxidase inhibitors or tricyclic antidepressants may intensify anticholinergic effects. Caution is advised when using Trihexyphenidyl HCI with neuroleptics due to an increased risk of tardive dyskinesia. Dosages of Trihexyphenidyl HCI and levodopa may need to be adjusted during combined therapy to manage drug-induced involuntary movements.
Common side effects include dry mouth, blurred vision, dizziness, mild nausea, and nervousness, which may subside with continued use. Rare side effects include suppurative parotitis, skin rashes, paralytic ileus, hallucinations, delusions, and cognitive dysfunctions. Other potential adverse effects include constipation, urinary retention, tachycardia, pupil dilation, and exacerbation of Parkinsonism symptoms after abrupt withdrawal. Neuroleptic Malignant Syndrome and angle-closure glaucoma, with associated blindness, have been reported.
Overdose symptoms include signs of atropine intoxication, such as dilated pupils, dry skin, tachycardia, elevated temperature, urinary retention, and neuropsychiatric symptoms like delirium and hallucinations. Treatment involves symptomatic and supportive care, including gastric lavage and respiratory support if necessary.
Store at temperatures not exceeding 30°C in a dry place, protected from light.