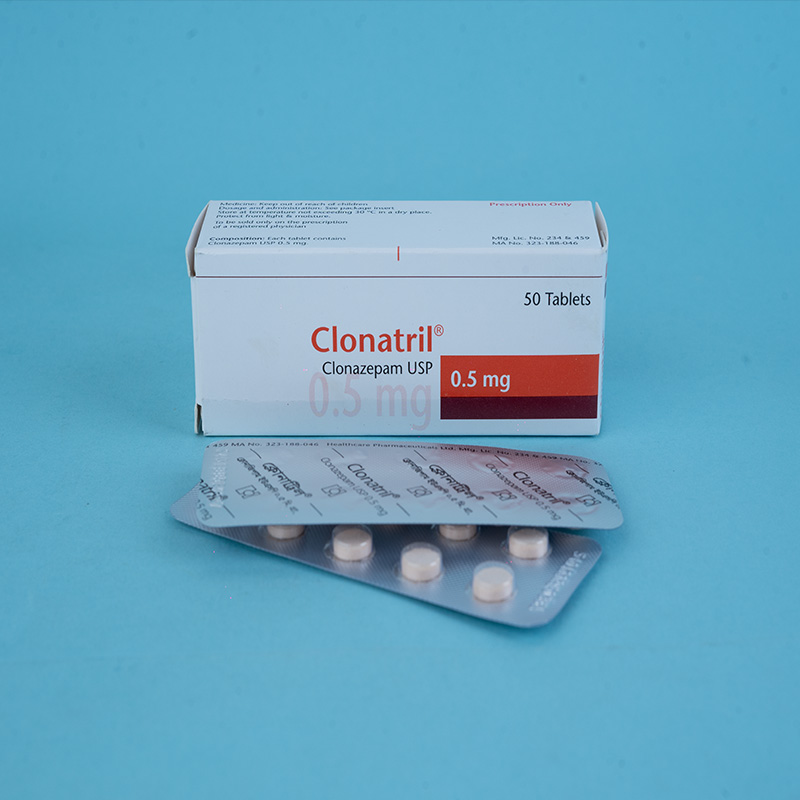
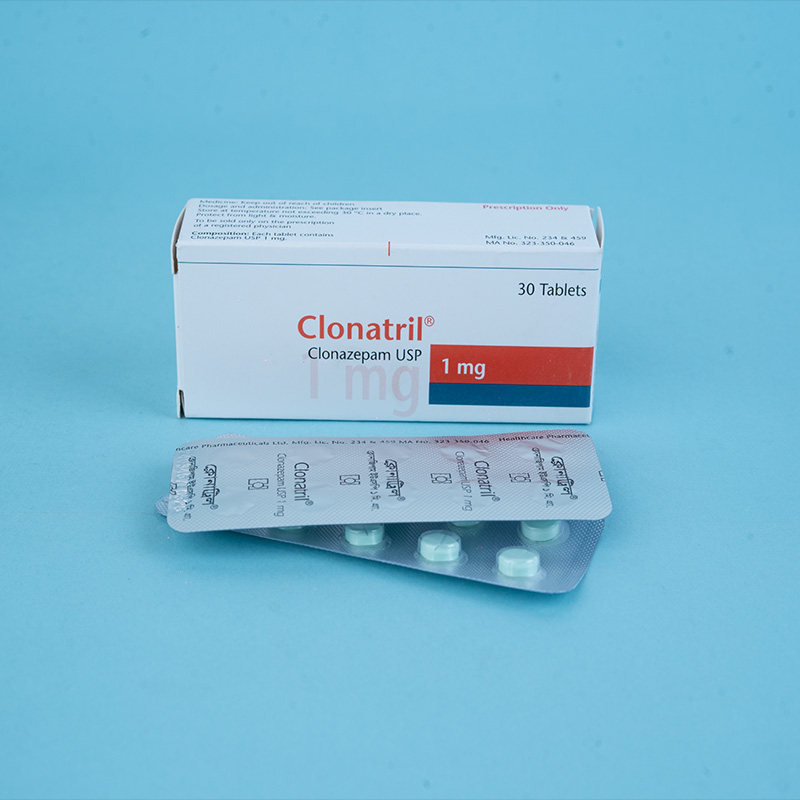
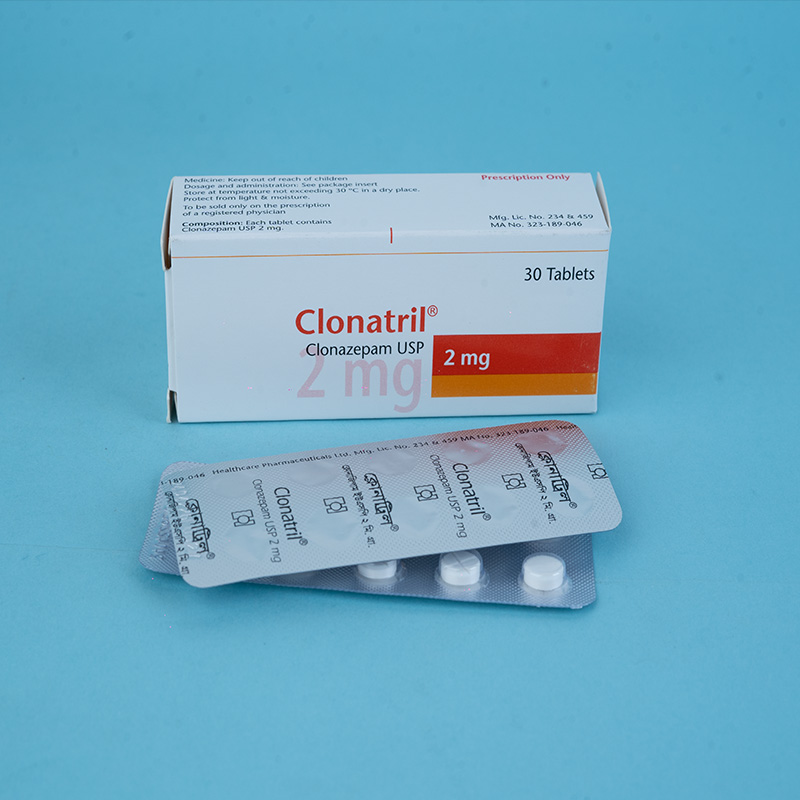
Clonazepam has pharmacological properties characteristic of the benzodiazepine class of drugs. It exhibits sedative, hypnotic, and anticonvulsant properties. Its basic anticonvulsive properties are similar to those of other benzodiazepines.
Anxiety and panic disorders, with or without agoraphobia. Epilepsy and other seizure disorders.
Standard Dosage: The dosage of Clonatril® must be individually adjusted according to the patient's clinical response and tolerance. It is generally administered as a low-dose, single-drug therapy in new, non-therapy-resistant cases.
Oral Treatment in Epilepsy: To minimize adverse reactions at the beginning of therapy, the daily dose should be increased progressively until the appropriate maintenance dose is reached.
Infants and children under 10 years (or up to 30 kg): Initial dose: 0.01-0.03 mg/kg daily. Maintenance dose: 0.05-0.1 mg/kg daily.
Children over 10 years (or over 30 kg) and adults: Initial dose: 1-2 mg daily. Maintenance dose: 1.5-3 mg daily for children aged 10-16 years, and 2-4 mg daily for adults.
Once the maintenance dose is reached, the daily amount may be given as a single dose in the evening. The maximum therapeutic dose for adults is 20 mg daily.
Panic Disorder: Clonazepam is typically started at 0.25-0.5 mg twice daily, gradually increasing by 0.25-0.5 mg every 3-5 days. Most patients are maintained on 1-3 mg/day in divided doses (BID), with some requiring 6-8 mg/day.
Gradual tapering of 0.25-0.5 mg per week is recommended when discontinuing the drug to prevent withdrawal symptoms.

Clonatril® may be used with other antiepileptic agents, but dosages must be adjusted to minimize sedation and other side effects. Concurrent use with liver enzyme inducers (e.g., barbiturates, hydantoins, carbamazepine) may accelerate its metabolism. The combination with valproic acid may cause petit mal status epilepticus. Alcohol should be strictly avoided, as it can alter the effects of the drug and increase side effects.
Symptoms: Symptoms range from drowsiness and light-headedness to coma, respiratory depression, and circulatory collapse. Serious outcomes are rare unless other substances are involved.
Management includes gastric lavage, supportive measures, and emergency care. Hypotension can be treated with sympathomimetic agents. The benzodiazepine antagonist flumazenil is not recommended for epilepsy patients, as it may provoke seizures.
Do not use this medicine beyond the expiry date indicated on the packaging.
Store at a temperature not exceeding 30°C and protect from light.