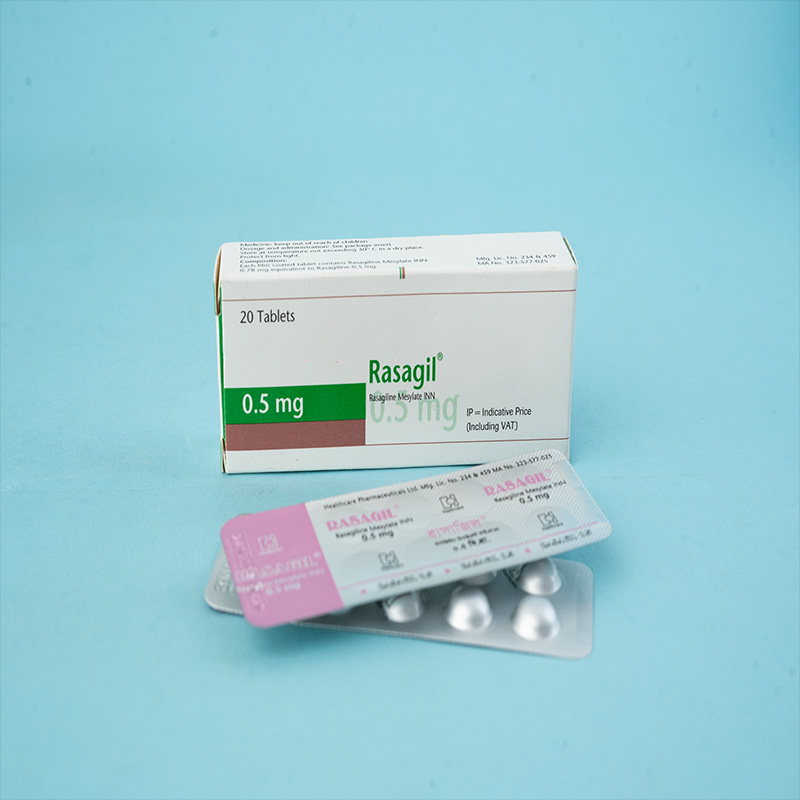
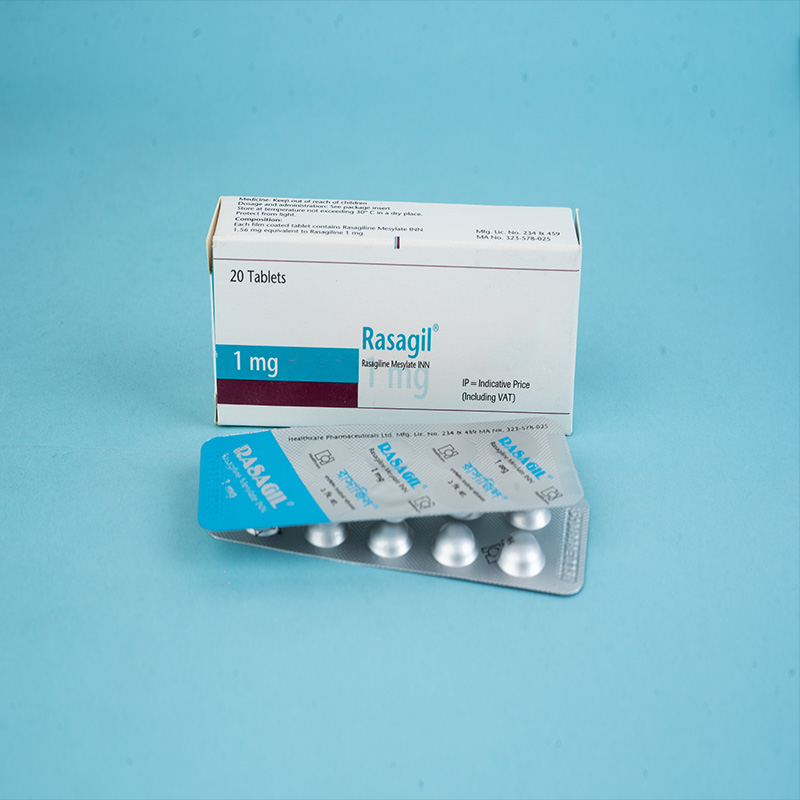
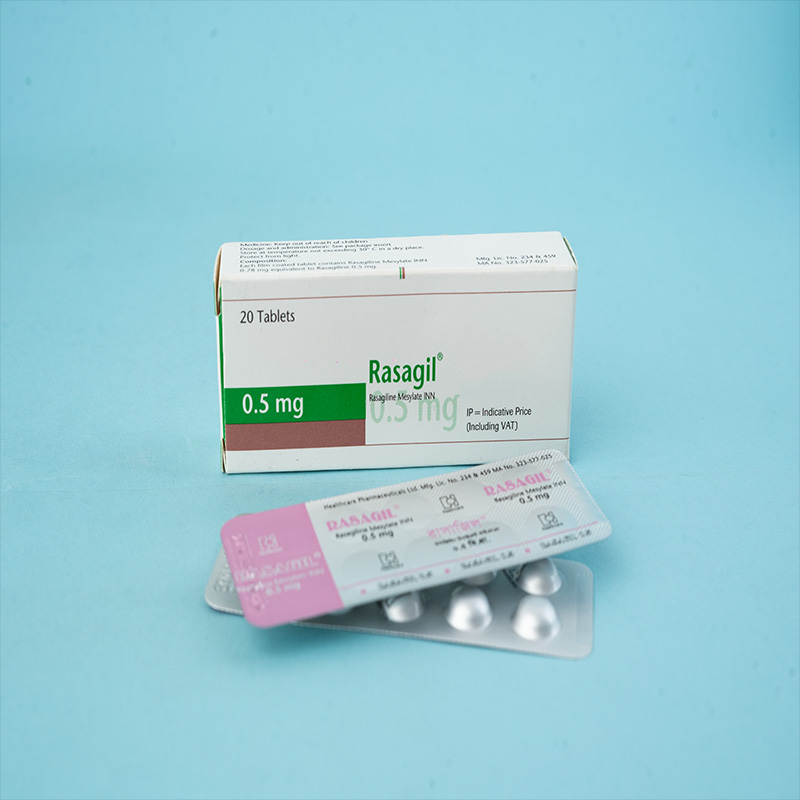
Rasagil® (Rasagiline tablet) is indicated for:
Monotherapy or adjunct therapy in the treatment of Parkinson's disease (PD).
Monotherapy or Adjunct Therapy (no levodopa): The recommended dose is 1 mg orally once daily.
Patients on levodopa:
The recommended starting dose is 0.5 mg once daily.
If tolerated but insufficient clinical response is seen, the dose may be increased to 1 mg once daily.
A reduction in levodopa dosage may be considered based on individual response.
The recommended dose of Rasagil® should not exceed 1 mg once daily due to the risk of hypertension.
Hypertension: Exacerbation of hypertension may occur. If sustained, medication adjustments may be needed. Tyramine-rich foods should be avoided due to the risk of severe hypertension from tyramine interaction, particularly at high levels of intake.
Serotonin Syndrome: This can occur when Rasagil® is combined with antidepressants (e.g., SSRIs, SNRIs, tricyclics, and tetracyclics) or nonselective MAOIs (e.g., phenelzine, tranylcypromine).
Ciprofloxacin or other CYP1A2 Inhibitors: Patients taking these should not exceed a 0.5 mg dose of Rasagil® once daily.
Hepatic Impairment: For mild hepatic impairment, the dose should be limited to 0.5 mg once daily.Rasagil® should not be used in patients with moderate or severe hepatic impairment.
Concomitant Use with Certain Medications: Avoid using Rasagil® with fluoxetine or fluvoxamine. At least 5 weeks should elapse between stopping fluoxetine and starting Rasagil®.
At least 14 days should pass between stopping Rasagil® and starting fluoxetine or fluvoxamine. Avoid the combination with dextromethorphan or sympathomimetics like ephedrine and pseudoephedrine.
Melanoma Risk: There have been cases of melanoma observed. However, the higher risk of skin cancer is thought to be associated with Parkinson's disease rather than Rasagil®.
See Contraindications & Precautions section for detailed drug interactions.
Pregnancy: Rasagil® is categorized as Pregnancy Category C. It should only be used during pregnancy if the benefits outweigh the risks to the fetus.
Lactation: It is unknown whether Rasagil® is excreted in human milk. Caution is advised when administering it to breastfeeding mothers.
The safety and effectiveness of Rasagil® in pediatric patients have not been established.
There are no significant differences in the safety profile between geriatric and nongeriatric patients.
Common side effects may include:
Central Nervous System: Joint pain, mild headache, dizziness, spinning sensation.
Dermatologic: Mild skin rash, hair loss.
Gastrointestinal: Dry mouth, constipation, diarrhea, stomach upset, vomiting.
Other: Loss of appetite, weight loss, numbness or tingling sensation.
Signs of overdose may include:
Drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonos, convulsions, and coma.
Other symptoms: Rapid and irregular pulse, hypertension, hypotension, vascular collapse, precordial pain, respiratory depression, hyperpyrexia, diaphoresis, cool, clammy skin.
Treatment:
There is no specific antidote for Rasagil® overdose.
Provide respiratory support, including airway management, oxygen supplementation, and mechanical ventilation as required.
Monitor body temperature closely, as intensive management of hyperpyrexia may be necessary.
Dietary tyramine restriction should be followed for several weeks to prevent hypertensive reactions.
Contact a poison control center for updated treatment guidelines.
Store at temperatures not exceeding 30°C in a dry place. Protect from light.