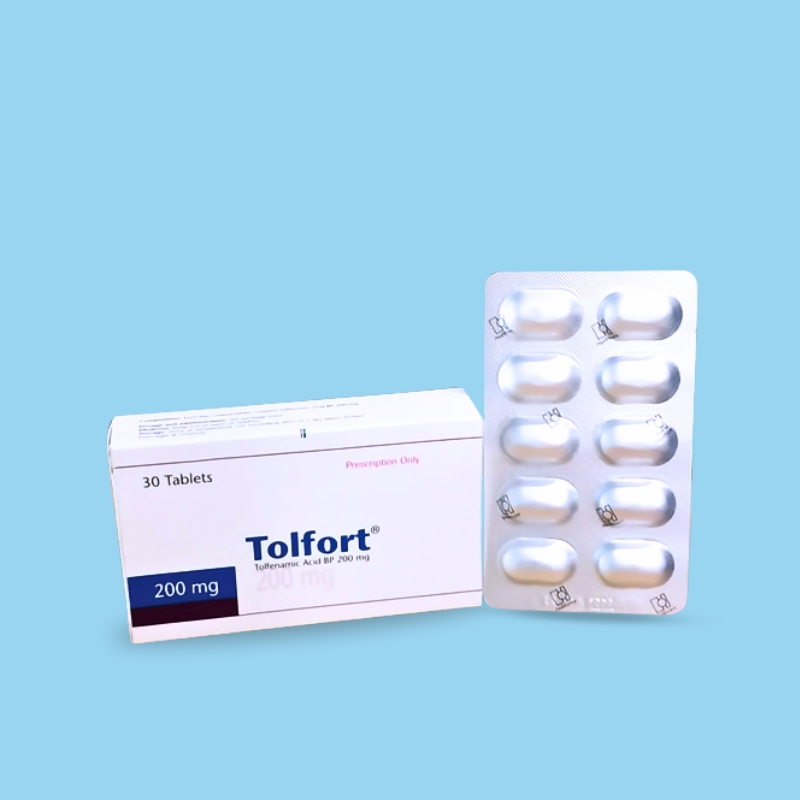
Tolfenamic acid is a non-steroidal anti-inflammatory drug (NSAID) which exerts anti-inflammatory and analgesic activities. The anti-inflammatory activity is mainly due to an inhibition of cyclooxygenase which leads to a subsequent reduction in the synthesis of prostaglandins.
• Tolfort (Tolfenamic acid BP 200mg) is indicated in acute migraine.
• It is also indicated for pain and swelling associated with musculoskeletal conditions such as rheumatoid arthritis, osteoarthritis and ankylosing spondylitis.
Adults: 200 mg tablets can be taken orally with or without food when the first symptoms of migraine appear. The treatment can be repeated once after 1-2 hours if a satisfactory response is not obtained.
Children: Not recommended for use in patients less than 18 years of age.
Elderly: Physician will prescribe a suitable dose.
Tolfenamic acid is contraindicated in patients with:
Hypersensitivity to tolfenamic acid or to any of the excipients. ⚫ Hypertension, severe heart failure, hepatic failure, renal failure, asthma.
• History of gastrointestinal bleeding or perforation, systemic lupus erythematosus.
Tolfenamic acid is well tolerated at the recommended dosage. The following undesirable effects have been observed:
Nausea, vomiting, diarrhoea, flatulence, constipation, dyspepsia, abdominal pain, hematemesis, ulcerative stomatitis, and Crohn's disease; Abnormal liver function, hepatitis and jaundice. Nephrotoxicity in various forms, nephrotic syndrome and renal failure; Thrombocytopenia, neutropenia, agranulocytosis, aplastic anaemia and haemolytic anaemia. Photosensitivity, visual disturbances, headaches, depression, confusion, hallucinations, tinnitus, vertigo, tremor, euphoria, dizziness, malaise, fatigue and drowsiness.
At high doses and in long term treatment, oedema, hypertension and cardiac failure have been reported. This product should be discontinued at the first appearance of skin rash, hypersensitivity, GI bleeding or ulceration. The use of tolfenamic acid may impair female fertility and is not recommended in women attempting to conceive.
If symptoms aggravate then activated charcoal should be considered within one hour of ingestion.
Inhibition of prostaglandin synthesis may adversely affect the pregnancy and/or the embryo/fetal development. During the first and second trimester of pregnancy, Tolfenamic acid should not be given unless clearly necessary. During the third trimester of pregnancy tolfenamic acid should not be used.
Tolfenamic acid should not be used in breast-feeding mothers.

Co-administration with Anti-coagulants, Diuretics, Anti-hypertensives, Lithium, Methotrexate, Ciclosporin, Mifepristone, Corticosteroids, Quinolone antibiotics, Anti-platelet agents and selective serotonin reuptake inhibitors (SSRIs) is not recommended. COMMERICAL PACK
Tolfort Tablet 200 mg: Each box contain 3 Blisters of 10 tablets in alu-alu blister pack.
Store at temperature not exceeding 30°C in a dry place. Protect from light and moisture.
Medicine: Keep out of reach of children