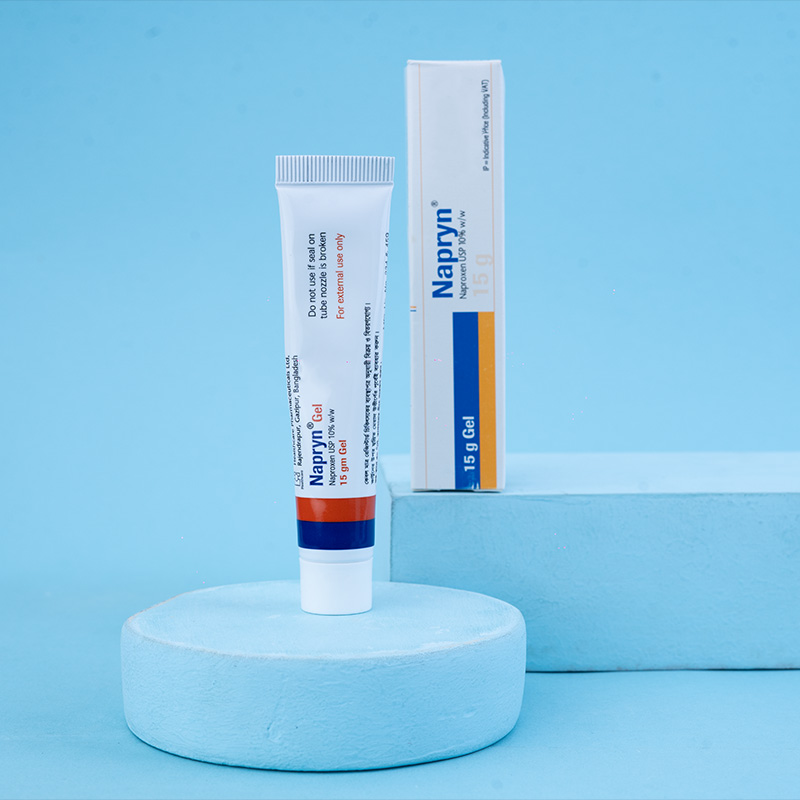
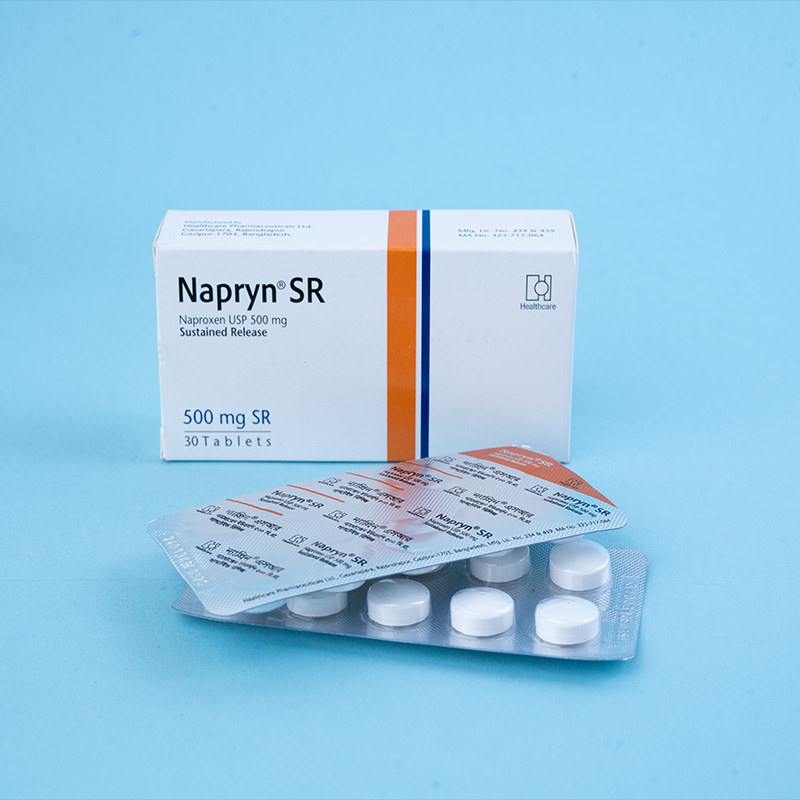
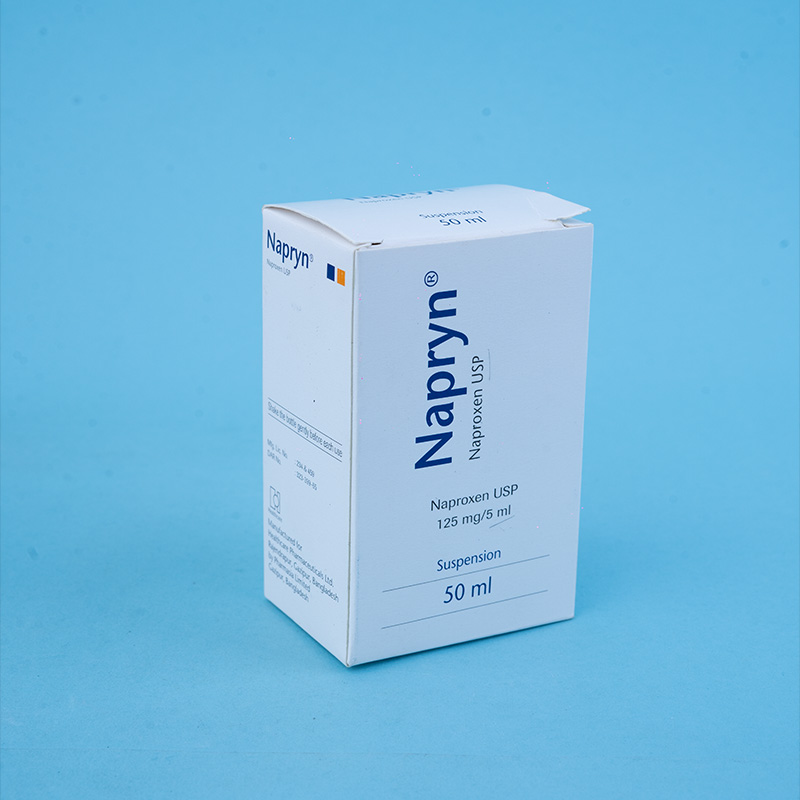
Napryn® (naproxen) is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic, anti-inflammatory, and antipyretic properties. It is a propionic acid derivative related to the arylacetic acid class of drugs. Chemically known as (+)-6-methoxy-alpha-methyl-2-naphthaleneacetic acid, naproxen has demonstrated strong anti-inflammatory effects in both human clinical studies and animal test systems. Additionally, it possesses notable analgesic and antipyretic actions by inhibiting prostaglandin synthesis.
Napryn® is indicated for the treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and juvenile rheumatoid arthritis. It is also used for tendonitis, bursitis, acute gout, pain management, primary dysmenorrhea, and migraine treatment and prophylaxis.
For naproxen tablets, pain relief typically begins within one hour. A lower dose is recommended for elderly patients and those with renal or hepatic impairment. Napryn® is not advised for patients with a creatinine clearance below 10 ml/min due to the risk of metabolite accumulation. The tablets can be taken on an empty stomach or with meals/antacids.
Adults with Chronic Conditions (Osteoarthritis, Rheumatoid Arthritis, Ankylosing Spondylitis, Chronic Pain with Inflammation): The recommended dose is 250 mg or 500 mg twice daily (morning and evening) or 500-1000 mg once daily. The dosage may be adjusted based on clinical response. If needed, the dose can be increased to 1500 mg per day under careful monitoring.
Acute Conditions (Analgesia, Dysmenorrhea, Acute Musculoskeletal Conditions, Acute Pain with Inflammation): The initial dose is Napryn® 500 mg, followed by 250 mg every 6-8 hours as required.
Acute Gout: The initial dose is 750 mg, followed by 250 mg every 8 hours until symptoms subside.
Migraine: For acute migraine, the recommended dose is 750 mg at the onset, followed by an additional 250-500 mg throughout the day if needed, with at least 30 minutes between doses. For migraine prophylaxis, the dose is 500 mg twice daily. If no improvement is observed within 4-6 weeks, treatment should be discontinued.
For children with juvenile rheumatoid arthritis, the recommended dose is 10 mg/kg/day in two divided doses every 12 hours. Use in children under 2 years is not recommended.
For Naproxen Gel, apply 2-6 times daily as required. It is not recommended for children.
Napryn® is contraindicated in patients with known hypersensitivity to naproxen. It should not be used in individuals who experience asthma, rhinitis, or nasal polyps after taking aspirin or other NSAIDs. Patients with active peptic ulcer disease or gastrointestinal bleeding should avoid naproxen. It is also not recommended for children under 2 years, as safety has not been established in this age group.
Possible gastrointestinal effects include abdominal pain, constipation, diarrhea, dyspepsia, heartburn, nausea, and stomatitis. Central nervous system effects may include dizziness, drowsiness, headache, lightheadedness, and vertigo. Dermatologic reactions can include itching, skin eruptions, and excessive sweating. Other reported side effects include hearing disturbances (tinnitus), visual disturbances, dyspnea, edema, palpitations, and increased thirst.
Gastrointestinal Risks: Naproxen may cause gastrointestinal irritation, ulceration, or bleeding, which can occur without warning. Patients with a history of gastrointestinal disease should use it under close supervision.
Renal Effects: Naproxen can impair renal function, leading to conditions such as renal failure or nephrotic syndrome. Patients with kidney disease should use it with caution.
Hematological Effects: Naproxen decreases platelet aggregation and prolongs bleeding time. Patients with bleeding disorders or those taking anticoagulants should be closely monitored.
Hepatic Effects: Severe liver reactions, including jaundice and hepatitis, have been reported.
Elderly Patients: Older adults may be at greater risk of adverse effects and should use the lowest effective dose.
NSAID Combination: Concurrent use with other NSAIDs is not recommended due to an increased risk of adverse effects.
Naproxen should not be used during pregnancy unless absolutely necessary, as it can delay labor and affect fetal cardiovascular function. It is not recommended during labor and delivery due to potential complications. Naproxen has been found in breast milk at low concentrations, so use in nursing mothers is not advised.
Antacids and Cholestyramine may delay naproxen absorption but do not affect its overall bioavailability.
Food can also delay absorption without impacting the total drug effect.
Plasma Albumin Binding: Naproxen is highly protein-bound; dose adjustments may be required for patients taking hydantoins, sulfonamides, or sulfonylureas.
Methotrexate: Naproxen can reduce methotrexate clearance, increasing toxicity.
Beta-blockers & Diuretics: Naproxen may reduce the effectiveness of beta-blockers and inhibit the natriuretic effect of diuretics.
Lithium: Naproxen may lead to increased plasma lithium levels.
Store at an appropriate temperature to maintain product stability.