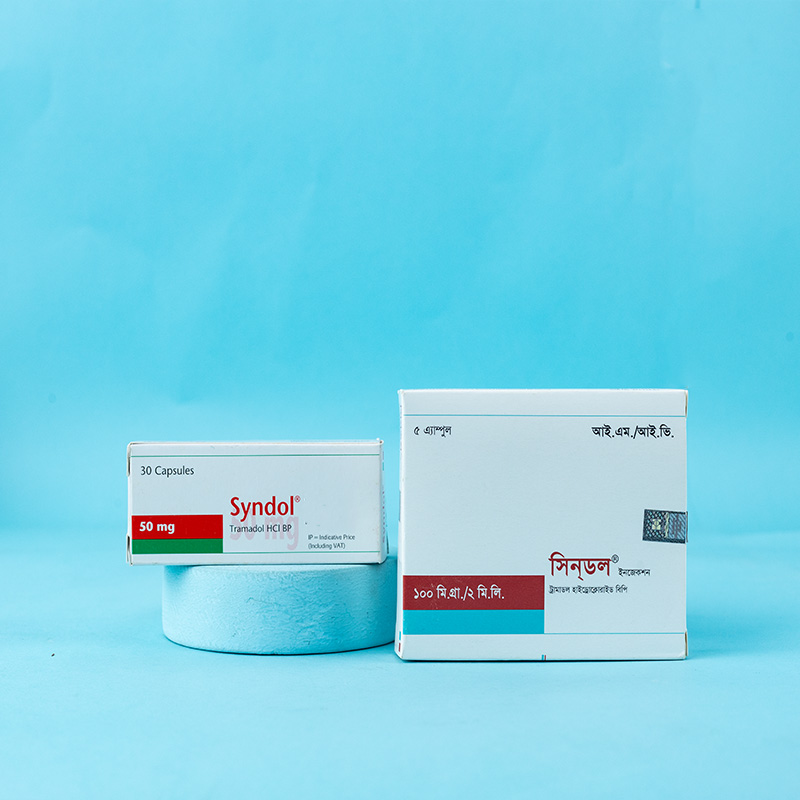
Syndol® Capsule: Each capsule contains Tramadol Hydrochloride BP 50 mg.
Syndol® 2 ml Injection: Each milliliter of the injection contains Tramadol Hydrochloride BP 50 mg.
Syndol® is a centrally acting opioid analgesic. It is a non-selective agonist at µ, δ, and κ opioid receptors, with a higher affinity for the µ receptor. Additional mechanisms contributing to its analgesic effect include the inhibition of neuronal reuptake of noradrenaline and the enhancement of serotonin release.
Syndol® is indicated for the management of moderate to severe pain.
Capsules:
The usual oral dose is 50-100 mg every 4-6 hours.
For chronic painful conditions, an initial dose of 50 mg is recommended.
The dose and frequency depend on the severity of the pain.
The total daily oral dose should not exceed 400 mg.
Administer by intravenous (IV), intramuscular (IM) injection (over 2-3 minutes), or intravenous infusion, at a dose of 50-100 mg every 4-6 hours.
For postoperative pain: An initial dose of 100 mg, followed by 50 mg every 10-20 minutes during the first hour if necessary, with a total maximum of 250 mg in the first hour (including the initial dose). Subsequently, administer 50-100 mg every 4-6 hours, not exceeding a daily maximum of 600 mg.
Syndol® is contraindicated in patients with:
Hypersensitivity to Tramadol or any of its components.
Acute intoxication with alcohol, opioids, hypnotics, centrally acting analgesics, or psychotropic drugs.
Common side effects include:
Nausea, vomiting (particularly during initial stages), constipation, and drowsiness.
Larger doses may cause respiratory depression and hypotension.
Other side effects include dry mouth, sweating, headache, vertigo, tachycardia, palpitations, hypothermia, hallucinations, dysphoria, mood changes, urticaria, and pruritus.
Children: For children above one year of age, a single dose of 1-2 mg/kg body weight is appropriate for the injectable form. Other forms are not recommended for children under 12 years due to insufficient safety and efficacy data.
Elderly: No dosage adjustment is typically required for patients aged 65-75 unless they have renal or hepatic impairment.
Pregnancy: Categorized as Pregnancy Category C. Do not use in pregnant women prior to or during labor unless the potential benefits outweigh the risks. The safety of Syndol® in pregnancy has not been established.
Lactation: Tramadol is not recommended for obstetric preoperative medication or post-delivery analgesia in nursing mothers, as its safety for infants and newborns has not been studied.
Use with caution in patients with a history of epilepsy or susceptibility to seizures.
When administered with anesthetics or alcohol, Tramadol may cause respiratory depression. Use cautiously in patients at risk for this condition.
Exercise caution in patients with increased intracranial pressure or head trauma.
Tramadol is not recommended for opioid-dependent patients, as they may experience withdrawal symptoms.
Syndol® interacts with CNS depressants, MAO inhibitors, cimetidine, and alcohol.
Drug Interactions:
Syndol® interacts with CNS depressants, MAO inhibitors, cimetidine, and alcohol.