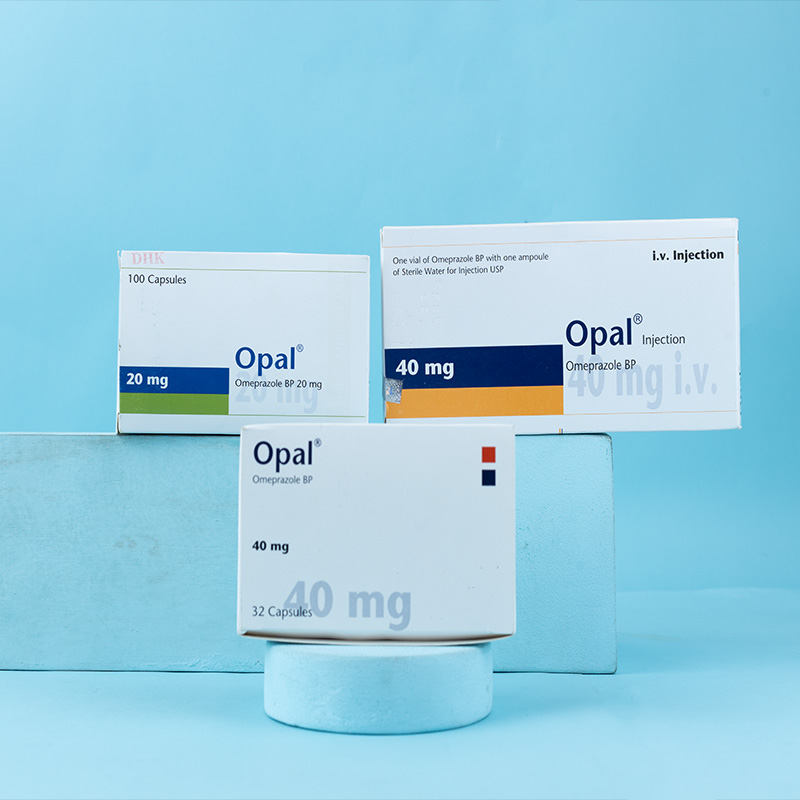
Opal® 20 mg capsules: Each capsule contains Omeprazole BP 20 mg as enteric-coated pellets.
Opal® 40 mg capsules: Each capsule contains Omeprazole BP 40 mg as enteric-coated pellets.
Opal® 40 mg i.v. injection: Each vial contains Omeprazole BP 40 mg (as Omeprazole sodium BP lyophilized sterile powder), and each ampoule contains 10 ml of sterile water for injection USP.

Opal® is indicated for:
Treatment of gastro-oesophageal reflux disease (GERD).
Peptic ulcer disease.
Eradication of Helicobacter pylori in peptic ulceration (triple therapy).
Acid-related dyspepsia.
NSAID-associated duodenal or gastric ulcers and gastroduodenal erosions.
Zollinger-Ellison Syndrome.

Opal® (Omeprazole) inhibits gastric acid secretion by irreversibly blocking the enzyme hydrogen/potassium adenosine triphosphatase (H+/K+ATPase), also known as the proton pump, in gastric parietal cells. Oral dosing with Opal® produces gastric acid secretion inhibition within 1-2 hours of the first dose. Maximum effect is achieved within 4 days of treatment, after which the degree of acid inhibition remains constant.

Omeprazole is rapidly absorbed, and absorption is not affected by food.
Omeprazole is acid-labile, and its absorption is dose-dependent.
Bioavailability may be increased in elderly patients and those with impaired hepatic function.
Omeprazole is metabolized almost completely in the liver by cytochrome P450 and is rapidly eliminated in the urine.
The elimination half-life is approximately 0.5 to 3 hours, but its effect on acid secretion is much longer, allowing for once-daily dosing.
Omeprazole is approximately 95% bound to plasma proteins.

Capsules:
GERD: 20-40 mg daily for 4 to 12 weeks. Maintenance therapy: 20 mg daily.
Peptic ulcer disease: 20 mg once daily (40 mg in severe cases). Duration: 4 weeks for duodenal ulcers, 8 weeks for gastric ulcers. Maintenance dose: 20 mg once daily.
H. pylori eradication: 40 mg daily in divided doses, combined with antibiotics (triple therapy).
NSAID-associated ulcers: 20 mg daily for treatment or prophylaxis in susceptible patients.
Zollinger-Ellison syndrome: Initial dose of 60 mg once daily; adjust individually as needed (up to 120 mg three times daily). For doses above 80 mg, divide and administer twice daily.
Acid-related dyspepsia: 20 mg daily for 2-4 weeks.
Injection:
Duodenal ulcer, gastric ulcer, reflux oesophagitis: 40 mg once daily for patients unable to take oral medication. Switch to oral therapy after 2-3 days.
Zollinger-Ellison syndrome: Adjust dose individually. Intravenous treatment can be administered as an infusion over 20-30 minutes.

Elderly and hepatic impairment: Adjust dose; maximum daily dose: 20 mg.
Renal impairment: No dose adjustment required.

Reconstitute Opal® IV injection (40 mg) by adding 10 ml of the provided sterile water to the vial. Administer as a slow intravenous injection over at least 2.5 minutes, at a maximum rate of 4 ml per minute. Use the solution within 4 hours of reconstitution.

There are no known contraindications to the use of Omeprazole. However, before starting treatment in patients with gastric ulcers, malignancy should be ruled out, as Omeprazole may mask symptoms and delay diagnosis.

Safety during pregnancy has not been established, though animal studies show no teratogenic effects.
There is no information on Omeprazole’s passage into breast milk. If treatment is essential, breastfeeding should be discontinued.

Opal® is generally well-tolerated. Adverse reactions, though rare, include:
Skin reactions: Rash, urticaria, pruritus, photosensitivity, and, in isolated cases, erythema multiforme, angioedema, and alopecia.
Gastrointestinal: Diarrhea, constipation, nausea, vomiting, flatulence, abdominal pain, stomatitis, and candidiasis.
Neurological: Headache, dizziness, paraesthesia, somnolence, insomnia, and, rarely, mental confusion, agitation, or hallucinations in severely ill patients.
Other: Arthritic symptoms, myalgia, blurred vision, taste disturbances, peripheral edema, gynaecomastia, thrombocytopenia, and interstitial nephritis.

Delayed elimination: Omeprazole may delay the elimination of diazepam, phenytoin, and warfarin. Dose adjustment may be necessary.
No interaction evidence: With theophylline, propranolol, metoprolol, lidocaine, quinidine, amoxicillin, or antacids.
Increased bioavailability of digoxin: A 10% increase has been observed.
Combination therapy: Co-administration with clarithromycin and amoxicillin increases plasma levels of Omeprazole.

Store below 30 ºC in a dry place, protected from light. Keep out of reach of children.