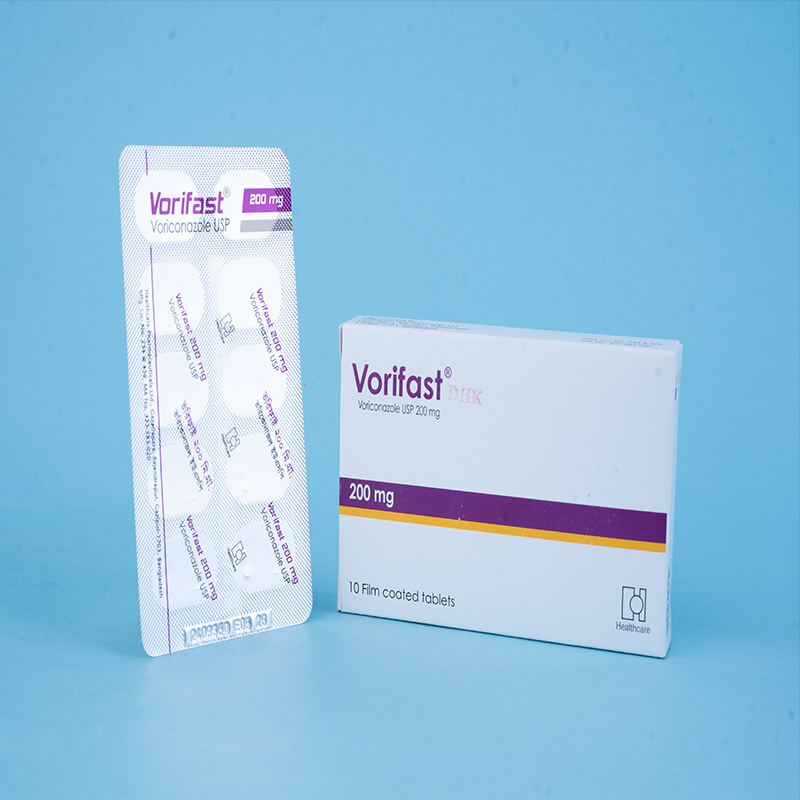
Vorifast® Tablet 50 mg: Each film-coated tablet contains Voriconazole USP 50 mg.
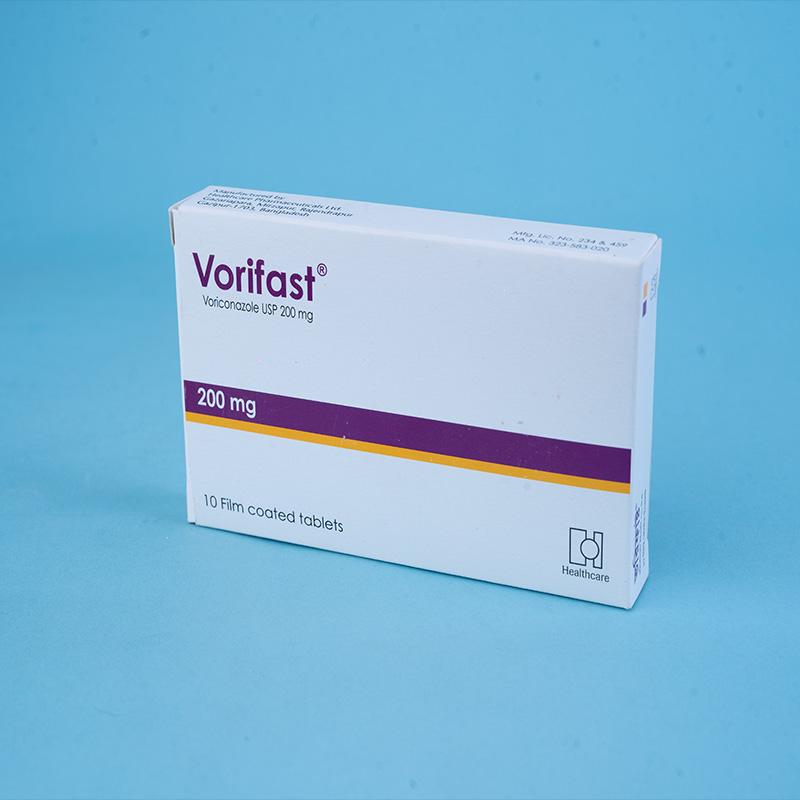
Vorifast® Tablet 200 mg: Each film-coated tablet contains Voriconazole USP 200 mg.
Vorifast® Injection 200 mg: Each vial contains Voriconazole USP 200 mg.
Vorifast® PFS: Each ml of oral suspension contains Voriconazole USP 40 mg.
Voriconazole is a broad-spectrum, triazole systemic antifungal agent.
The primary mode of action of Voriconazole is the inhibition of fungal cytochrome P450-mediated 14 alpha-lanosterol demethylation, an essential step in fungal ergosterol biosynthesis.
Voriconazole is indicated in adults and children aged 2 years and above for:
Treatment of invasive aspergillosis.
Treatment of candidaemia in non-neutropenic patients.
Treatment of fluconazole-resistant serious invasive Candida infections (including C. krusei).
Treatment of serious fungal infections caused by Scedosporium spp. and Fusarium spp. Voriconazole should be administered to patients with progressive, potentially life-threatening infections.
Prophylaxis of invasive fungal infections in high-risk allogeneic hematopoietic stem cell transplant (HSCT) recipients.
In Adults
Therapy must begin with the specified loading dose regimen (intravenous or oral) to achieve plasma concentrations close to steady state on day 1. Due to its high oral bioavailability (96%), switching between intravenous and oral administration is appropriate when clinically indicated.
Duration of Treatment
Treatment should be as short as possible, depending on clinical and mycological response. Long-term exposure (over 180 days) requires careful benefit-risk assessment.
Prophylaxis in Adults and Children
Prophylaxis begins on the day of transplant and can last up to 100 days, depending on the risk of invasive fungal infections (IFI) as defined by neutropenia or immunosuppression.
Dosage for Special Populations
Elderly: No dose adjustment is necessary.
Renal Impairment
The pharmacokinetics of Voriconazole are not affected by renal impairment; no dose adjustment is required for oral dosing.
Hepatic Impairment
Use the standard loading dose regimen, but halve the maintenance dose in patients with mild to moderate hepatic cirrhosis (Child-Pugh A and B). Voriconazole has not been studied in severe hepatic cirrhosis (Child-Pugh C).
Paediatric Population
Safety and efficacy in children under 2 years of age have not been established.
Tablets
Take at least one hour before or one hour after a meal.
Injection
Reconstitute and dilute before intravenous infusion.
PFS (Powder for Suspension)
Reconstitute before oral use. Take at least one hour before or two hours after a meal.
Reconstitution Directions
Injection
This product is for single use only; discard unused solution.
Remove the plastic cap from the vial and clean with an antiseptic swab.
Reconstitute the powder by adding 19 ml of Sterile Water for Injection using the provided syringe.
This yields 20 ml of clear concentrate at 10 mg/ml. Ensure complete dissolution (do not shake).
Dilution for Infusion
Infuse over 1–2 hours at a concentration of 5 mg/ml or less.
Adjust the volume based on the patient's weight and ensure the final concentration is between 0.5 mg/ml and 5 mg/ml.
Use a syringe to withdraw the required volume of concentrate and add it to the infusion bag.
Suspension
Shake the bottle to loosen the powder.
Add 25 ml of cooled boiled water (use the provided spoon for measurement).
Shake vigorously until fully mixed.
In case of overdose, haemodialysis may help remove Voriconazole and SBECD.
Voriconazole is contraindicated in patients with known hypersensitivity to the drug or other azoles.
Long-term treatment (over 180 days) requires careful benefit-risk assessment.
Physicians should limit exposure to Voriconazole where possible.
Common adverse reactions include:
Visual disturbances
Fever
Nausea, vomiting
Rash
Headache
Abnormal liver function tests
Tachycardia
Hallucinations
Voriconazole affects CYP3A4, CYP2C9, and CYP2C19 enzymes. Adjust dosage of co-administered drugs accordingly.
Monitor for interactions with phenytoin or efavirenz; dose adjustments may be necessary.
Pregnancy
Should not be used unless benefits outweigh risks to the fetus.
Lactation
The excretion of Voriconazole in breast milk is not studied; avoid use during breastfeeding.
Tablets and Injection: Store below 30°C in a dry place, protected from light and moisture.
Reconstituted Injection: Use immediately or refrigerate (2–8°C) for up to 24 hours.
PFS: Store below 30°C, protect from light. Do not freeze.
Tablets: Available in 50 mg and 200 mg in Alu-Alu blister packs of 10 tablets each.
Injection: Each box contains 1 vial of 200 mg, 1 ampoule of 20 ml Sterile Water for Injection, a sterile disposable syringe, butterfly needle, alcohol pad, and first-aid bandage.
PFS: Each box contains a 40 ml bottle of powder with a plastic spoon.
Keep out of reach of children.