LUZU (luliconazole) Cream, 1% is indicated for the topical treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organisms Trichophyton rubrum and Epidermophyton floccosum in patients 18 years of age and older.
For topical use only. LUZU Cream, 1% is not for ophthalmic, oral, or intravaginal use.When treating interdigital tinea pedis, a thin layer of LUZU Cream, 1% should be applied to the affected area and approximately 1 inch of the immediate surrounding area(s) once daily for two (2) weeks.When treating tinea cruris or tinea corporis, LUZU Cream, 1% should be applied to the affected area and approximately 1 inch of the immediate surrounding area(s) once daily for one (1) week.
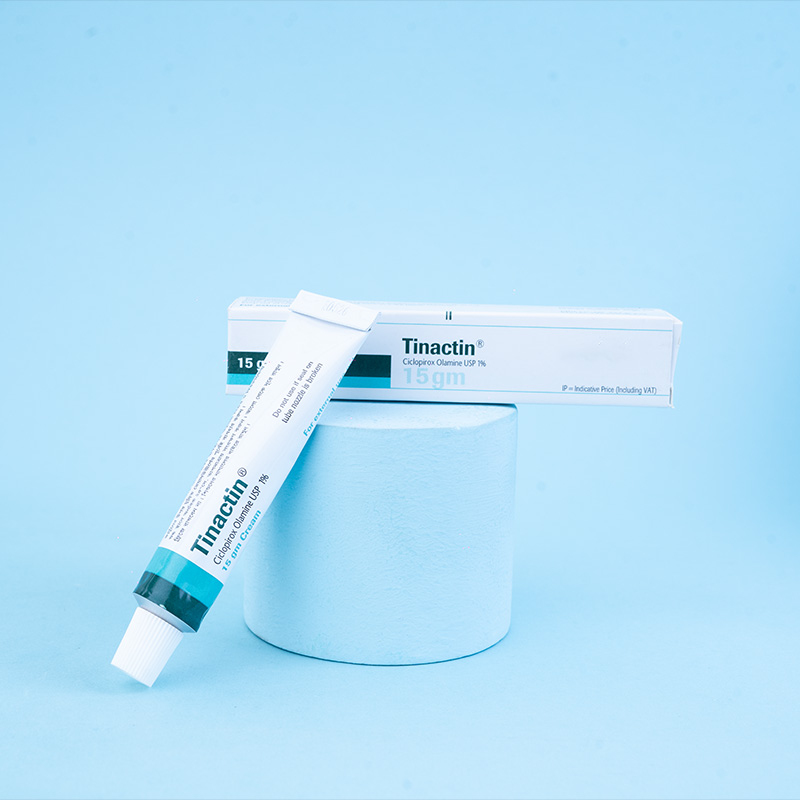
LUZU Cream, 1% is available as a cream formulation. Each gram of LUZU Cream, 1% contains 10 mg of luliconazole in a white cream base.
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In three Phase 3 clinical trials, 616 subjects were exposed to LUZU Cream, 1%, including 305 subjects with interdigital tinea pedis and 311 subjects with tinea cruris. Subjects with interdigital tinea pedis or tinea cruris applied LUZU Cream, 1% or vehicle cream once daily for 14 or 7 days, respectively, to the affected and adjacent areas.
During clinical trials with LUZU Cream, 1%, the most common adverse reactions were application site reactions, occurring in less than 1% of subjects in both the LUZU and vehicle arms. Most adverse reactions were mild in severity.
Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies of LUZU Cream, 1% in pregnant women. LUZU Cream, 1% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The animal multiples of human exposure calculations were based on daily dose body surface area (BSA) comparisons (mg/m²) for the reproductive toxicology studies described in this section and in Section 13.1. The Maximum Recommended Human Dose (MRHD) was set at 8 g of 1% cream per day (1.33 mg/kg/day for a 60 kg individual, equivalent to 49.2 mg/m²/day).
Systemic embryofetal development studies were conducted in rats and rabbits. Subcutaneous doses of 1, 5, and 25 mg/kg/day of luliconazole were administered during the period of organogenesis (gestational days 7–17) to pregnant female rats. No treatment-related effects on maternal toxicity or malformations were noted at 25 mg/kg/day (3 times the MRHD based on BSA comparisons). Increased incidences of skeletal variation (14th rib) were noted at 25 mg/kg/day. No treatment-related effects on skeletal variation were noted at 5 mg/kg/day (0.6 times the MRHD based on BSA comparisons).
Subcutaneous doses of 4, 20, and 100 mg/kg/day of luliconazole were administered during the period of organogenesis (gestational days 6–18) to pregnant female rabbits. No treatment-related effects on maternal toxicity, embryofetal toxicity, or malformations were noted at 100 mg/kg/day (24 times the MRHD based on BSA comparisons).
In a pre- and post-natal development study in rats, subcutaneous doses of 1, 5, and 25 mg/kg/day of luliconazole were administered from the beginning of organogenesis (gestation day 7) through the end of lactation (lactation day 20). In the presence of maternal toxicity, embryofetal toxicity (increased prenatal pup mortality, reduced live litter sizes, and increased postnatal pup mortality) was noted at 25 mg/kg/day. No embryofetal toxicity was noted at 5 mg/kg/day (0.6 times the MRHD based on BSA comparisons). No treatment effects on postnatal development were noted at 25 mg/kg/day (3 times the MRHD based on BSA comparisons).
Nursing Mothers: It is not known whether luliconazole is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when LUZU Cream, 1% is administered to women who are breastfeeding.
Pediatric Use: The safety and effectiveness of LUZU Cream, 1% in pediatric patients have not been established. The number of pediatric patients ≥12 years of age was too small to adequately assess safety and efficacy.
Geriatric Use
Of the total number of subjects in clinical studies of LUZU Cream, 1%, 8% were 65 and over, while 1.4% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Storage Conditions: Store at controlled room temperature, 20°C to 25°C (68°F to 77°F). Excursions are permitted between 15°C and 30°C (59°F to 86°F).
Keep Out of Reach of Children: Ensure the medication is stored in a safe place, away from children and pets.
Protect from Light and Moisture: Store in a dry place and keep the tube tightly closed when not in use.
Do Not Freeze: Avoid exposure to extreme temperatures.