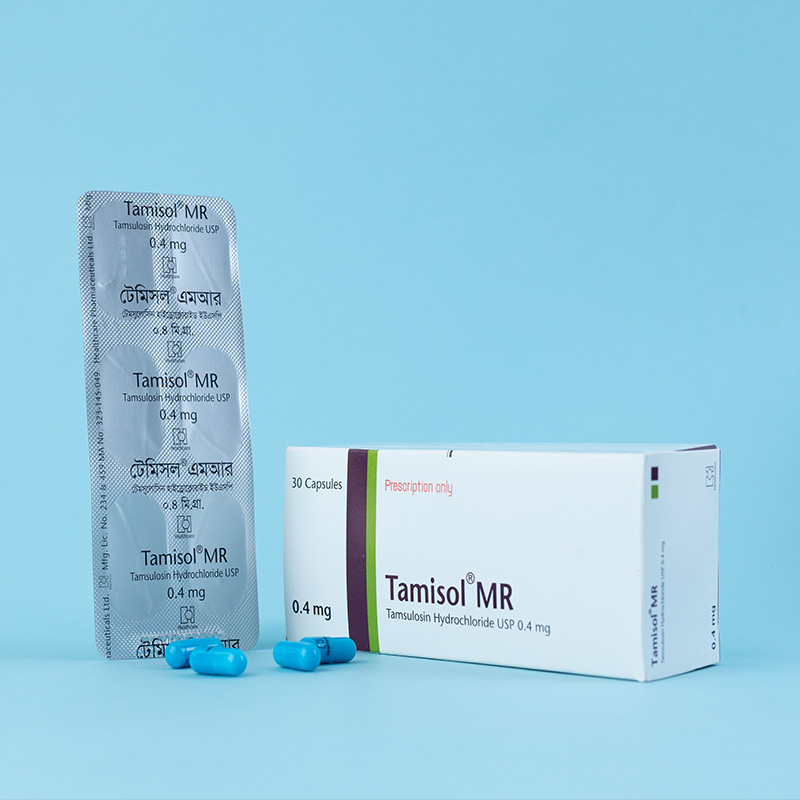
Each capsule contains pellets of modified-release Tamsulosin Hydrochloride USP 0.4 mg as the active ingredient.
Tamisol® MR is indicated for the treatment of functional symptoms of benign prostatic hyperplasia (BPH). It relaxes smooth muscle in BPH, leading to an increase in urinary flow rate and an improvement in obstructive symptoms.
The recommended dose is one capsule daily, taken after the same meal each day. The capsule should be swallowed whole and should not be crushed or chewed, as this may interfere with the modified release of the active ingredient
Tamisol® MR is contraindicated in patients with:
Hypersensitivity to Tamsulosin Hydrochloride or any other component of the product.
A history of orthostatic hypotension.
Severe hepatic insufficiency.
As with other alpha1-blockers, a reduction in blood pressure may occur in some individuals during treatment with Tamisol® MR, which can rarely result in syncope (fainting). At the first signs of orthostatic hypotension (such as dizziness or weakness), the patient should sit or lie down until symptoms subside.
Before initiating therapy with Tamisol® MR, the patient should be examined to rule out other conditions that may present with symptoms similar to benign prostatic hyperplasia.
The following adverse reactions have been reported with Tamsulosin Hydrochloride:
Common side effects: Dizziness, abnormal ejaculation.
Less frequent (1-2%): Headache, asthenia (weakness), postural hypotension, palpitations, and rhinitis.
Occasionally: Gastrointestinal symptoms such as nausea, vomiting, diarrhea, and constipation.
Hypersensitivity reactions: Rash, pruritus (itching), and urticaria (hives).
Other rare effects: Drowsiness, blurred vision, dry mouth, or edema, similar to other alpha-blockers.
No significant interactions have been observed when Tamsulosin Hydrochloride is taken with atenolol, enalapril, nifedipine, or theophylline. Cimetidine may increase plasma levels of Tamsulosin. Furosemide may decrease plasma levels, but dosage adjustments are not required, as levels remain within the normal range. Tamsulosin does not alter the free fractions of diazepam, propranolol, trichlormethiazide, or chlormadinone.
Not applicable, as Tamisol® MR is intended for male patients only.

No cases of acute overdose have been reported. However, an overdose may theoretically cause acute hypotension. In such cases, cardiovascular support should be provided.
Store at a temperature not exceeding 25ºC in a dry place. Protect from light.
Medicine: Keep out of reach of children.