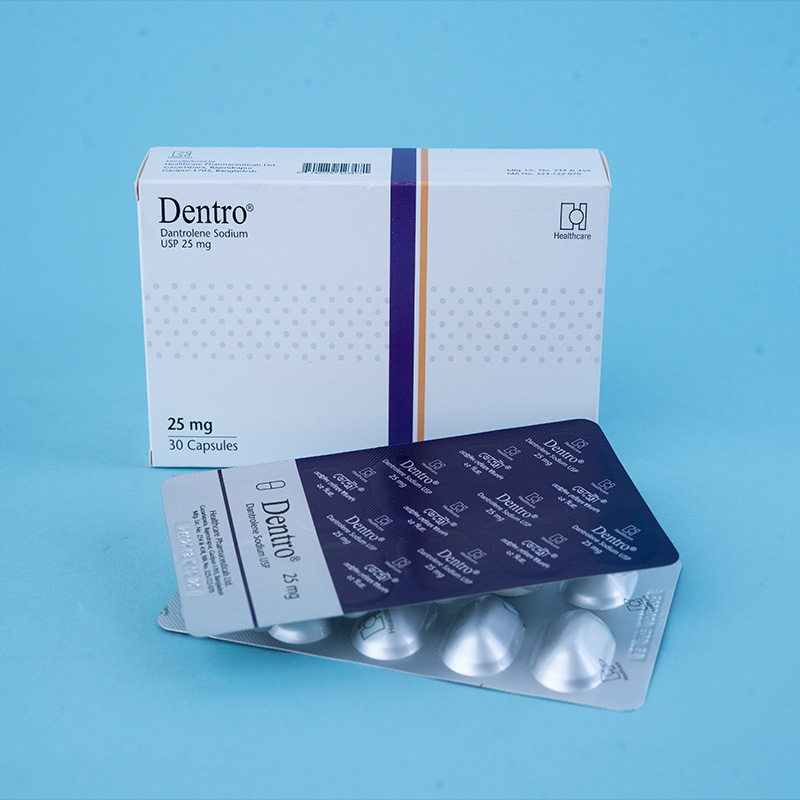
Dentro® Capsule 25 mg: Each capsule contains Dantrolene Sodium (Hydrate) USP 29.68 mg, equivalent to Dantrolene Sodium 25 mg.
Dentro® Capsule 50 mg: Each capsule contains Dantrolene Sodium (Hydrate) USP 59.36 mg, equivalent to Dantrolene Sodium 50 mg.
Dentro® produces muscle relaxation by affecting the contractile response of skeletal muscle at a site beyond the myoneural junction, directly on the muscle itself. In skeletal muscle, Dentro® dissociates excitation-contraction coupling, probably by interfering with the release of Ca²⁺ from the sarcoplasmic reticulum.
For Chronic Spasticity:
Adults: The following gradual titration schedule is suggested, as some patients may not respond until higher doses are reached. Each dosage level should be maintained for seven days to assess response. If no further benefit is observed at the next higher dose, the dosage should be reduced to the previous lower dose.
Starting dose: 25 mg twice daily.
Can be increased by 25-50 mg per day per week.
Maximum accepted dosage: 400 mg per day.
Pediatric Patients:
0.5 mg/kg once daily for seven days.
Then 0.5 mg/kg three times daily for seven days.
Then 1 mg/kg three times daily for seven days.
Then 2 mg/kg three times daily.
Some patients may require therapy four times daily.
For Malignant Hyperthermia:Preoperative: 4 to 8 mg/kg/day in three or four divided doses for one to two days before surgery. The last dose should be given approximately 3 to 4 hours before surgery with a minimum amount of water.
Post-crisis follow-up: 4 to 8 mg/kg/day in four divided doses for one to three days to prevent recurrence of malignant hyperthermia.
Due to the potential for liver damage with long-term Dentro® use, therapy should be stopped if no benefits are observed within 45 days.
Patients should avoid driving or engaging in hazardous activities while taking Dentro®.
Caution should be exercised when combining Dentro® with tranquilizers.
Dentro® may cause photosensitivity; patients should limit sun exposure.
Serious liver disorders (fatal and non-fatal) may occur.
Liver function tests (SGOT, SGPT, alkaline phosphatase, total bilirubin) should be conducted before starting treatment and at appropriate intervals during therapy.
If symptoms of hepatitis or jaundice appear, Dentro® should be discontinued immediately.
In some cases, liver function abnormalities may reverse after stopping the drug.
Reintroducing Dentro® after liver issues should be done only in hospitalized patients with very small and gradually increasing doses, with frequent monitoring. Higher risk of liver damage exists in females and patients over 35 years of age.
Active hepatic disease (e.g., hepatitis, cirrhosis).
Use with caution in patients with impaired pulmonary function or severe cardiac dysfunction due to myocardial disease.
Use with caution in patients with a history of liver disease or dysfunction.
Most frequently reported side effects include: drowsiness, dizziness, weakness, malaise, fatigue, and diarrhea.
These effects are usually transient and occur early in treatment.
Starting with a low dose and increasing gradually can reduce side effects.
Diarrhea may be severe and require temporary withdrawal. If diarrhea recurs after reintroducing Dentro®, therapy should be permanently discontinued.
Pregnancy (Category C): Dentro® should be used only if the potential benefit outweighs the risk to the fetus.
Nursing Mothers: Dentro® should not be used while breastfeeding.
The long-term safety of Dentro® in children under 5 years has not been established.
Because adverse effects may appear over time, long-term use in pediatric patients should be carefully considered.
Drowsiness may occur, and combining Dentro® with CNS depressants (e.g., sedatives, tranquilizers) can increase drowsiness.
Hepatotoxicity risk is higher in women over 35 years of age taking estrogen therapy.