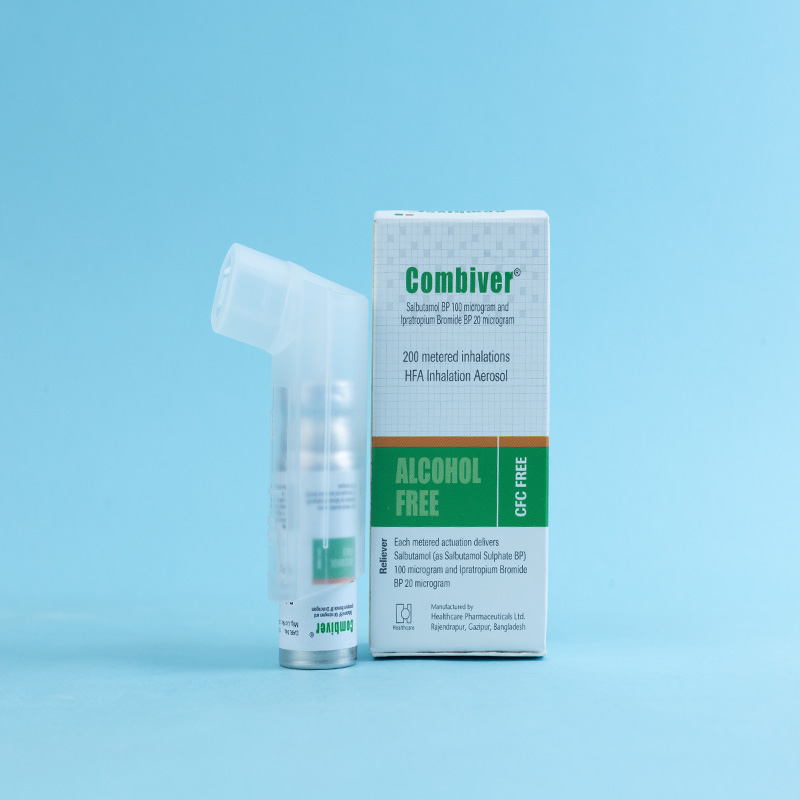
Combiver® inhaler is a pressurized metered-dose inhaler intended for oral inhalation only. It contains a combination of Salbutamol BP, a short-acting β₂-agonist bronchodilator, and Ipratropium Bromide BP, an anticholinergic bronchodilator. When used together, these two agents produce a greater bronchodilator effect than when used individually. This combination is designed to maximize the response to treatment in patients with chronic obstructive pulmonary disease (COPD) by reducing bronchospasm through two distinct mechanisms: anticholinergic (parasympatholytic) and sympathomimetic actions.

Combiver® inhaler is indicated as a bronchodilator for the treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD) in patients requiring regular treatment with both Salbutamol and Ipratropium. It is also beneficial for patients with asthma presenting COPD symptoms and those with a history of smoking exceeding 10 pack-years.

The dosage of Combiver® inhaler should be individualized based on the patient's needs.
Adults (including elderly patients and adolescents aged 12 years and above): 2 inhalations (puffs) four times a day. Additional inhalations may be taken if required; however, the total number of inhalations should not exceed 12 within 24 hours.
Children and pediatric patients: The safety and effectiveness of this medication in patients below 12 years of age have not been established.

This combination has not been studied in patients with hepatic or renal insufficiency. It should be used with caution in such patients.

Combiver® inhaler is contraindicated in patients with a history of hypersensitivity to any of its ingredients. It is also contraindicated in patients with hypertrophic obstructive cardiomyopathy, tachyarrhythmia, or hypersensitivity to atropine or its derivatives. Additionally, patients with a known hypersensitivity to soya lecithin or related food products, such as soybeans and peanuts, should avoid using this medication.

Since Combiver® inhaler contains Ipratropium Bromide, caution is advised in patients with narrow-angle glaucoma, prostatic hypertrophy, or bladder-neck obstruction. As it also contains Salbutamol Sulphate, a sympathomimetic amine, caution should be exercised in patients with convulsive disorders, hyperthyroidism, diabetes mellitus, or those unusually responsive to sympathomimetic amines. In some cases, β-adrenergic agents may cause significant but usually transient hypokalemia, which has the potential to produce adverse cardiovascular effects.
Paradoxical Bronchospasm
Combiver® inhaler may cause paradoxical bronchospasm, which can be life-threatening. If this occurs, the medication should be discontinued immediately, and an alternative treatment should be initiated. Paradoxical bronchospasm associated with inhaled medications often occurs with the first use of a new canister.
Cardiovascular Effects
Like other β-adrenergic agonists, Salbutamol Sulphate may cause significant cardiovascular effects in some patients, including increased pulse rate, blood pressure changes, or other symptoms. While these effects are uncommon at recommended doses, if they occur, discontinuation of the medication may be necessary. β-adrenergic agents have also been reported to cause electrocardiogram (ECG) changes, such as T-wave flattening, QTc prolongation, and ST-segment depression. Therefore, caution is advised in patients with cardiovascular conditions, including coronary insufficiency, cardiac arrhythmia, and hypertension.
Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions, although rare, may occur following administration of Ipratropium Bromide or Salbutamol Sulphate. Reported reactions include urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema.

Since the Combiver® inhaler contains both Salbutamol and Ipratropium Bromide, adverse effects associated with each component may occur.
Salbutamol: Rare reports include mild tremors and headaches, which usually subside with continuous treatment. Transient muscle cramps have been reported very rarely. Hypersensitivity reactions, such as angioedema, urticaria, bronchospasm, hypotension, and collapse, have also been observed in very rare cases.
Ipratropium: Reported side effects include headaches, pain, influenza, coughing, pneumonia, chest pain, nausea, bronchitis, dyspnea, and lower airway bronchospasm. Upper respiratory symptoms such as pharyngitis, sinusitis, and rhinitis have also been reported.

Ipratropium: Pregnancy Category B – Animal studies have not shown teratogenic effects with Ipratropium Bromide.
Salbutamol: Pregnancy Category C – Salbutamol has been shown to be teratogenic in mice, but there are no well-controlled studies in pregnant women.
Since animal studies do not always predict human responses, this inhaler should be used during pregnancy only if the potential benefits outweigh the potential risks to the fetus. It is not known whether the active ingredients of this inhaler are excreted in human breast milk. Therefore, it should only be used by lactating mothers if the potential benefits outweigh the risks to the infant.

This inhaler has been used concomitantly with other COPD treatments, including sympathomimetic bronchodilators, methylxanthines, and steroids, without adverse interactions. However, caution should be exercised in the following cases:
Anticholinergic agents: Co-administration with other anticholinergic drugs may lead to additive effects.
β-Adrenergic agents: Increased risk of cardiovascular side effects; caution is advised.
β-Receptor blockers: These may inhibit the effects of Salbutamol. β-blockers should be used cautiously in patients with hyperreactive airways.
Diuretics: Non-potassium-sparing diuretics may enhance the risk of hypokalemia.
Monoamine Oxidase Inhibitors (MAOIs) and Tricyclic Antidepressants: These may enhance the effects of β-adrenergic agonists and should be discontinued at least two weeks before starting this inhaler.

Overdose symptoms are expected to be primarily related to Salbutamol. Acute overdose with Ipratropium Bromide is unlikely due to its poor systemic absorption. Overdose symptoms may include anginal pain, hypertension, hypokalemia, and tachycardia (up to 200 beats per minute). In severe cases, cardiac arrest and even death have been reported due to the abuse of sympathomimetic aerosol medications. Hemodialysis is not an effective treatment for Salbutamol overdose. Instead, a selective cardiovascular β-receptor blocker, such as Metoprolol Tartrate, may be considered under medical supervision.

This is a pressurized canister—do not puncture, break, or incinerate, even if empty.
Avoid exposure to direct sunlight or heat. Store below 30ºC.
Keep away from eyes.
Keep out of reach of children.