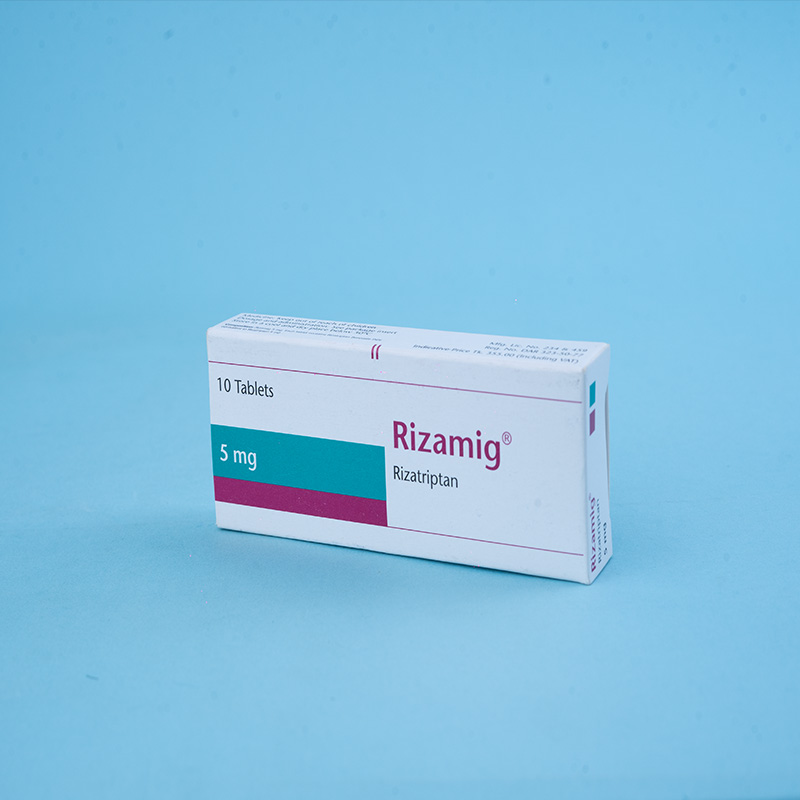
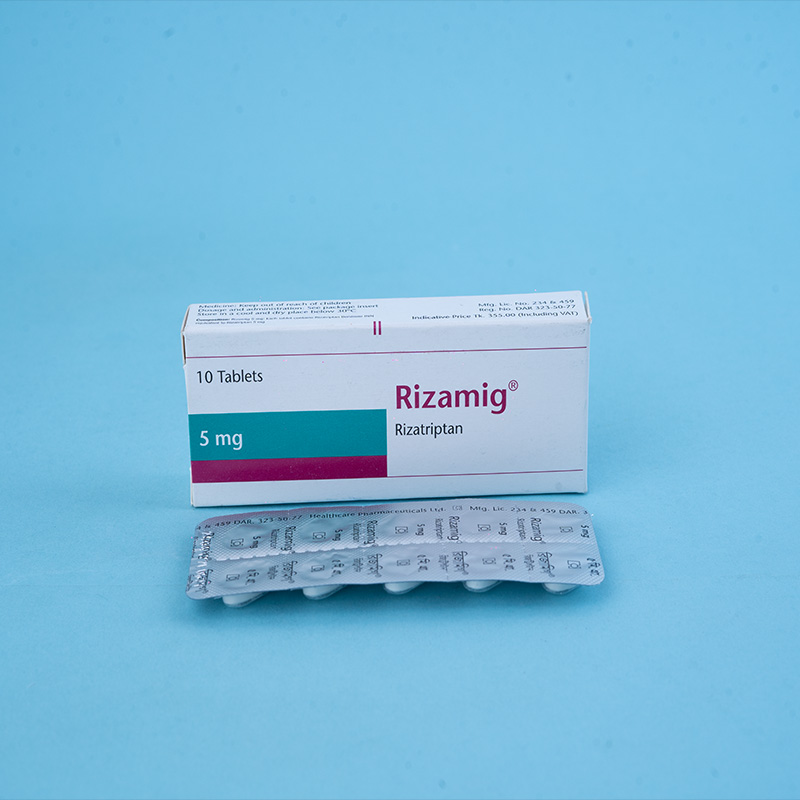
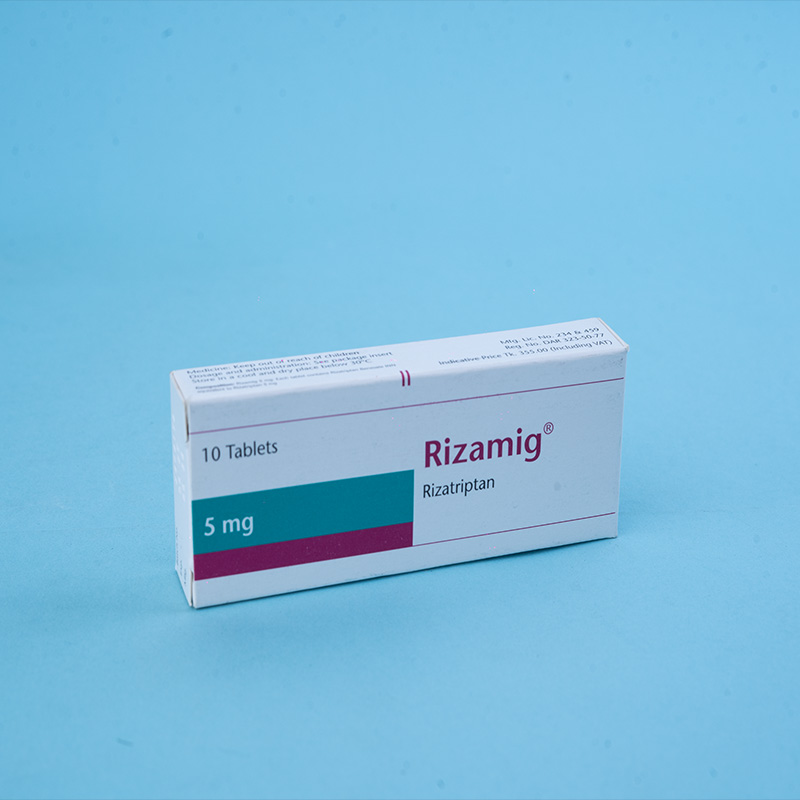
Active ingredient: Rizatriptan
Rizamig® 5 mg: Each tablet contains Rizatriptan Benzoate INN equivalent to Rizatriptan 5 mg.
Rizamig® is a selective 5-hydroxytryptamine 1B/1D (5-HT1B/1D) receptor agonist with anti-migraine effects.
Rizamig® is indicated for the acute treatment of migraine attacks, with or without aura, in adults.
Patients with ischemic heart disease (e.g., angina, myocardial infarction, or silent ischemia) or symptoms consistent with ischemic heart disease or coronary artery vasospasm.
Patients with uncontrolled hypertension.
Hypersensitivity to Rizatriptan or any inactive ingredients.
Use Rizamig® only when there is a clear diagnosis of migraine.
Do not use Rizamig® in patients with documented ischemic or vasospastic coronary artery disease.
Drugs in this class may cause coronary artery vasospasm. Patients experiencing angina-like symptoms after dosing should be evaluated for coronary artery disease (CAD) before additional doses and monitored electrocardiographically.
Use with caution in patients with moderate hepatic insufficiency.
If there is no response to the first dose, reconsider the migraine diagnosis before administering a second dose.
Propranolol: Use Rizatriptan 5 mg in patients taking propranolol.
Ergotamine and 5-HT1 Agonists: Rizatriptan should not be used within 24 hours of ergotamine-containing medications or other 5-HT1 agonists.
Monoamine Oxidase Inhibitors (MAOIs): Rizatriptan should not be administered with MAO-A inhibitors or non-selective MAO inhibitors.
Pregnancy: There are no adequate studies in pregnant women. Use Rizatriptan during pregnancy only if the potential benefit justifies the risk to the fetus.
Nursing Mothers: It is not known if Rizatriptan is excreted in human milk. Use with caution in nursing women.
Pediatric Use: The safety and effectiveness of Rizatriptan in children under 18 years of age have not been established.
Elderly Use: Clinical experience in elderly patients is limited. No significant differences in efficacy or adverse effects have been noted between younger and elderly patients.

Rare cardiac events, such as coronary artery vasospasm, myocardial infarction, ventricular tachycardia, and ventricular fibrillation, have been reported, typically in patients with risk factors for CAD.
Other reported side effects include chills, heat sensitivity, facial edema, abdominal distention, fever, orthostatic effects, syncope, and swelling.
Overdose symptoms include dizziness, somnolence, and potentially hypertension or other cardiovascular symptoms.
Treatment involves gastrointestinal decontamination (gastric lavage and activated charcoal).
Packs: Rizamig® 5 mg – Each box contains 1x10 tablets in a blister pack.
Storage: Store in a cool, dry place below 30°C. Protect from light.
Caution: Keep out of reach of children.