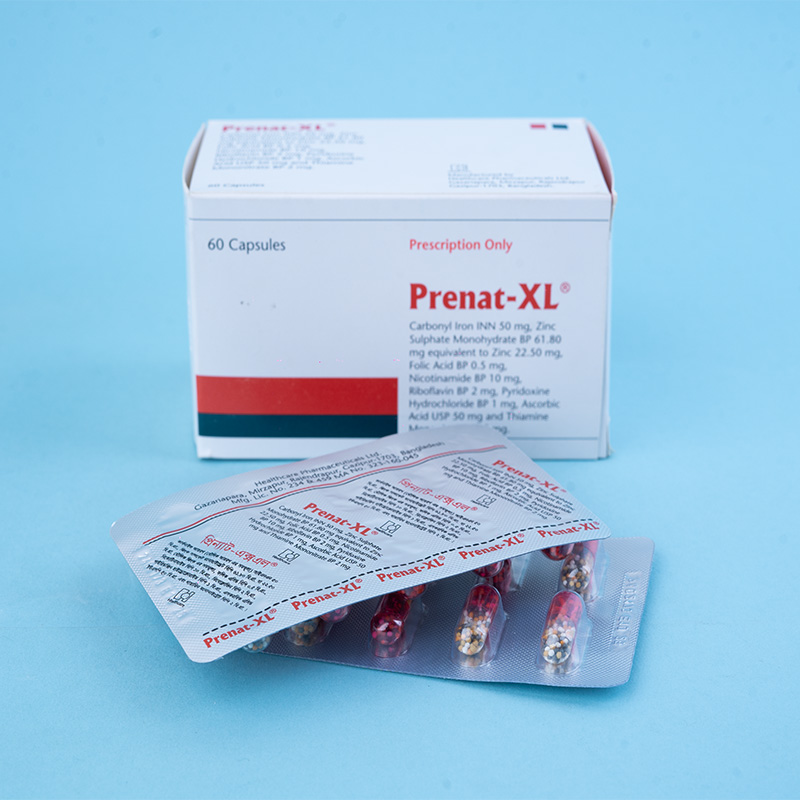
Each capsule contains elemental iron 50 mg (as carbonyl iron INN), zinc sulfate monohydrate USP 61.80 mg (equivalent to 22.50 mg zinc), and folic acid BP 500 micrograms. Carbonyl iron is 98% pure iron, virtually non-toxic, and easily absorbed by the body.

Prenat CI® is indicated for the treatment and prophylaxis of iron, folic acid, and zinc deficiency, especially during pregnancy and lactation.

Adults: 1 capsule daily. In more severe cases, 2 capsules daily may be required.
Elderly: Dosage as above.
Children (aged over 1 year): 1 capsule daily. The capsule may be opened, and the pellets mixed with soft, cool food, but they must not be chewed.

Do not use in patients hypersensitive to any of the components of the product or those with iron overload.

Care should be taken in patients who may develop iron overload, such as those with haemochromatosis, hemolytic anemia, and red cell aplasia. Failure to respond to treatment may indicate other causes of anemia, and further investigation is recommended.
In patients with renal failure, there is a risk of zinc accumulation.

Administration of iron during the first trimester requires definite evidence of iron deficiency. Prophylaxis of iron deficiency, where an inadequate diet calls for supplementary zinc and folic acid, is justified during the remainder of pregnancy.

Carbonyl iron is less likely to cause constipation and diarrhea, which are significant problems with iron salt supplements. Traditional forms of iron are poorly absorbed (less than 1%). It is the unabsorbed iron that has a toxic effect on the body. The irritation in the lower intestine causes constipation and, in some cases, diarrhea, as the body tries to rid itself of excess iron. Dark stools are common during iron therapy. Nausea and other symptoms of gastrointestinal irritation, such as anorexia, vomiting, discomfort, constipation, and diarrhea, are sometimes encountered. Zinc may also produce gastrointestinal upset. There have been rare reports of allergic reactions.

Iron overdose is dangerous, particularly in children, and requires immediate attention. Gastric lavage should be carried out in the early stages, or if this is not possible, vomiting should be induced. These procedures should not be undertaken if signs of the corrosive effects of zinc are present. In that case, give oral desferrioxamine (2 g for a child or 5 g for an adult) and a demulcent. If serum iron levels at 4 hours or more post-ingestion are over 5 mg/l in a child or 8 mg/l in an adult, or if the patient is in a coma, intravenous desferrioxamine should be used. Zinc sulfate in gross overdosage is corrosive. Symptoms are those of gastrointestinal irritation, leading in severe cases to hemorrhage, corrosion of the mucosa, and possible later stricture formation. Gastric lavage or emesis should be avoided. Demulcent, such as milk, should be given. Chelating agents such as dimercaprol, penicillamine, or edetic acid have been recommended. Symptomatic and supportive measures should be provided as required.

Store in a cool and dry place below 30°C and protect from light. Keep out of children's reach to avoid accidental iron poisoning.