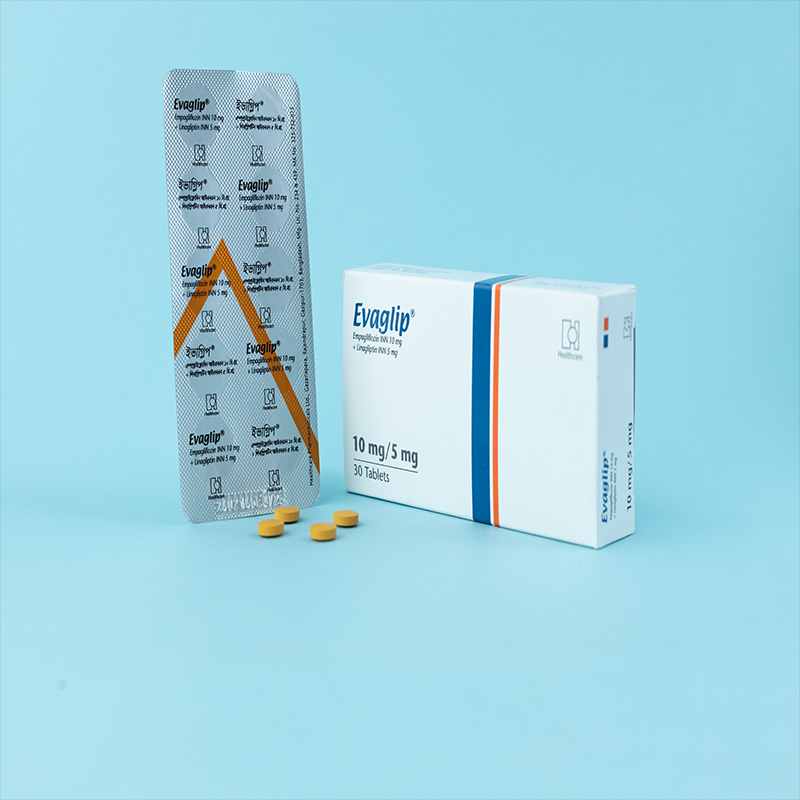
EVAGLIP® Tablet 10 mg/5 mg: Each film-coated tablet contains Empagliflozin INN 10 mg and Linagliptin INN 5 mg.
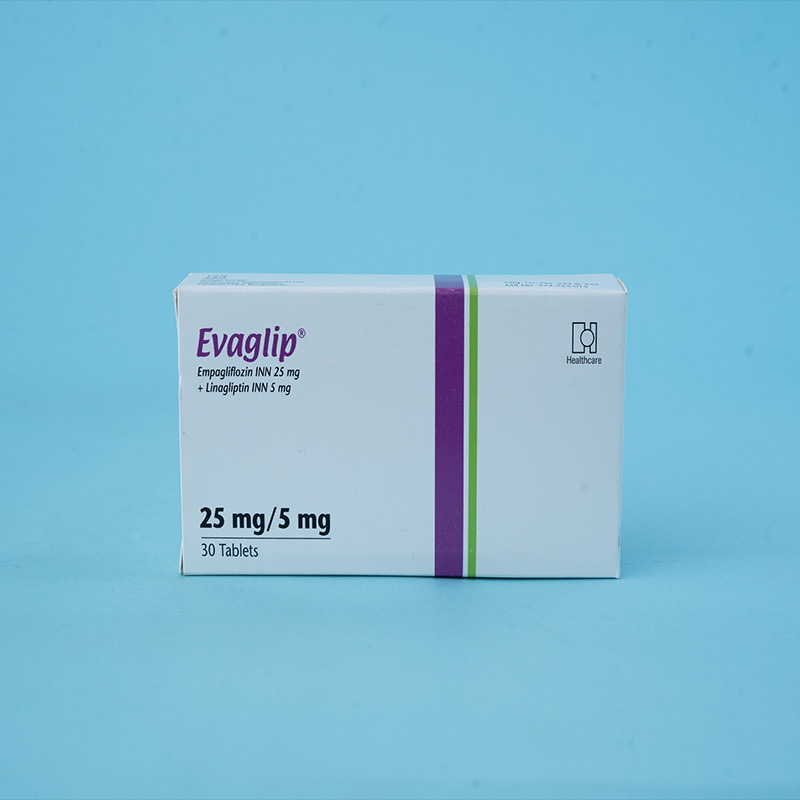
EVAGLIP® Tablet 25 mg/5 mg: Each film-coated tablet contains Empagliflozin INN 25 mg and Linagliptin INN 5 mg.

EVAGLIP® is a combination of empagliflozin and linagliptin. Both are oral diabetes medicines that help control blood sugar levels.

As an adjunct to diet and exercise, EVAGLIP® is used to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both empagliflozin and linagliptin is appropriate.

The recommended dose of EVAGLIP® is 10 mg empagliflozin/5 mg linagliptin once daily in the morning, with or without food.
In patients tolerating EVAGLIP®, the dose may be increased to 25 mg empagliflozin/5 mg linagliptin once daily.

EVAGLIP® is contraindicated in patients with:
A history of hypersensitivity reactions to linagliptin, empagliflozin, or any components of the medication.
Severe renal impairment, end-stage renal disease, or dialysis.

Pancreatitis: Post-marketing reports indicate a risk of acute pancreatitis, including fatal cases. Discontinue EVAGLIP® if pancreatitis is suspected.
Hypotension: Assess and correct volume status before starting EVAGLIP®, especially in patients with renal impairment, low systolic blood pressure, elderly patients, or those on diuretics. Monitor for hypotension during therapy.
Renal Impairment: Monitor renal function regularly, with more frequent checks in patients with an eGFR below 60 mL/min/1.73 m².
Hypoglycemia: Reduce the dose of insulin or insulin secretagogues when starting EVAGLIP® to decrease the risk of hypoglycemia.
Genital Mycotic Infections: Monitor and treat as needed.
Urinary Tract Infections: Monitor and treat as needed.
Hypersensitivity: If hypersensitivity reactions occur, discontinue EVAGLIP® immediately, investigate other causes, and initiate alternative diabetes treatment.
Increased LDL-C: Monitor and treat elevated LDL-C levels as appropriate.
Macrovascular Outcomes: No clinical studies conclusively demonstrate that EVAGLIP® reduces macrovascular risk.

Possible side effects include:
Urinary tract infections, upper respiratory tract infections, common cold symptoms, genital yeast infections, increased urination, joint pain, nausea, runny or stuffy nose, and diarrhea.

Pregnancy: There are no adequate and well-controlled studies of EVAGLIP® in pregnant women. Use only if the potential benefit justifies the potential risk to the fetus.
Lactation: Discontinue use in nursing mothers as it is unknown if the medication is excreted in human milk.

The safety and effectiveness of EVAGLIP® in pediatric patients have not been established.

Elderly patients may experience a higher incidence of adverse reactions related to volume depletion and reduced renal function.

Adverse reactions related to reduced renal function are more common in patients with impaired renal function.

Empagliflozin:
When co-administered with diuretics, it may increase urine volume and frequency of voids, enhancing the potential for volume depletion.
Co-administration with insulin or insulin secretagogues increases the risk of hypoglycemia.
Urine glucose monitoring is not recommended as empagliflozin increases urinary glucose excretion, leading to positive urine glucose tests.
Linagliptin:
The efficacy of linagliptin may be reduced when taken with strong P-gp or CYP3A4 inducers. Use alternative treatments in such cases.
OVERDOSE
In the event of an overdose, seek medical attention immediately.

Store at a temperature not exceeding 30°C in a dry place. Protect from light and moisture.
Medicine: Keep out of reach of children.